BORIC ACID 99%, kg
124.99 €
00262002
Orthoboric acid, CAS 10043-35-3, boron trihydroxide, INCI BORIC ACID, Orthoboric acid, Boracic acid, Sassolite, Optibor, Borofax, Trihydroxyborane, Acide borique.
Parameter | Attribute |
Boric acid | Ortoboric acid, boric trihydroxide, Trihidro borate, Sassolite, Optibor, Borofax, Boroxyborane, Trihydroxyborane, Boron(III) hydroxide, Boron trihydroxide, Boron trihydride, Acide borique |
Formula | B(OH)3; H3BO3 |
Structure | 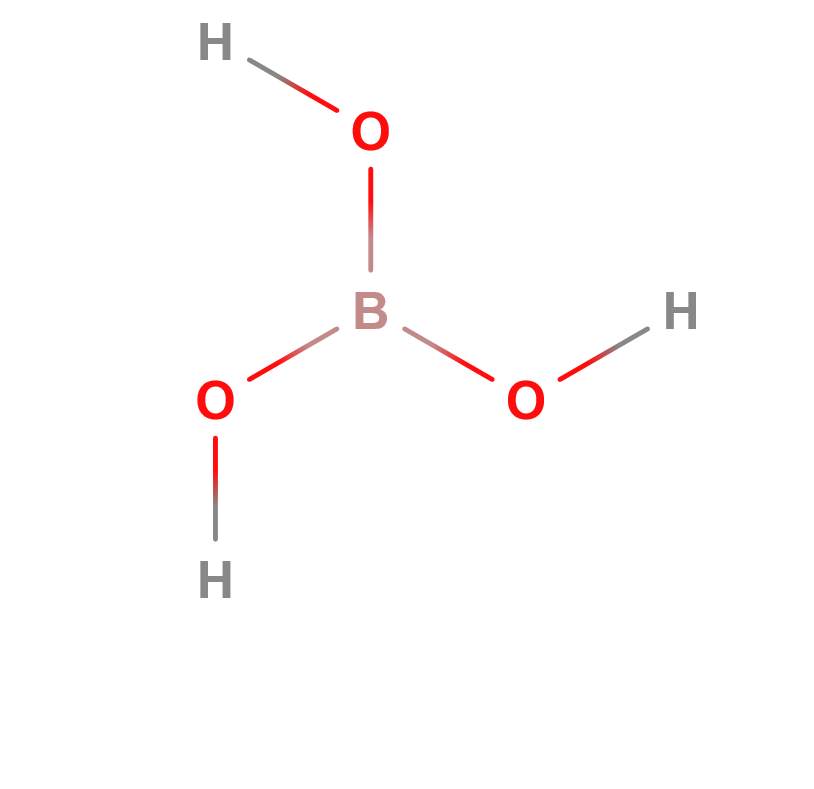 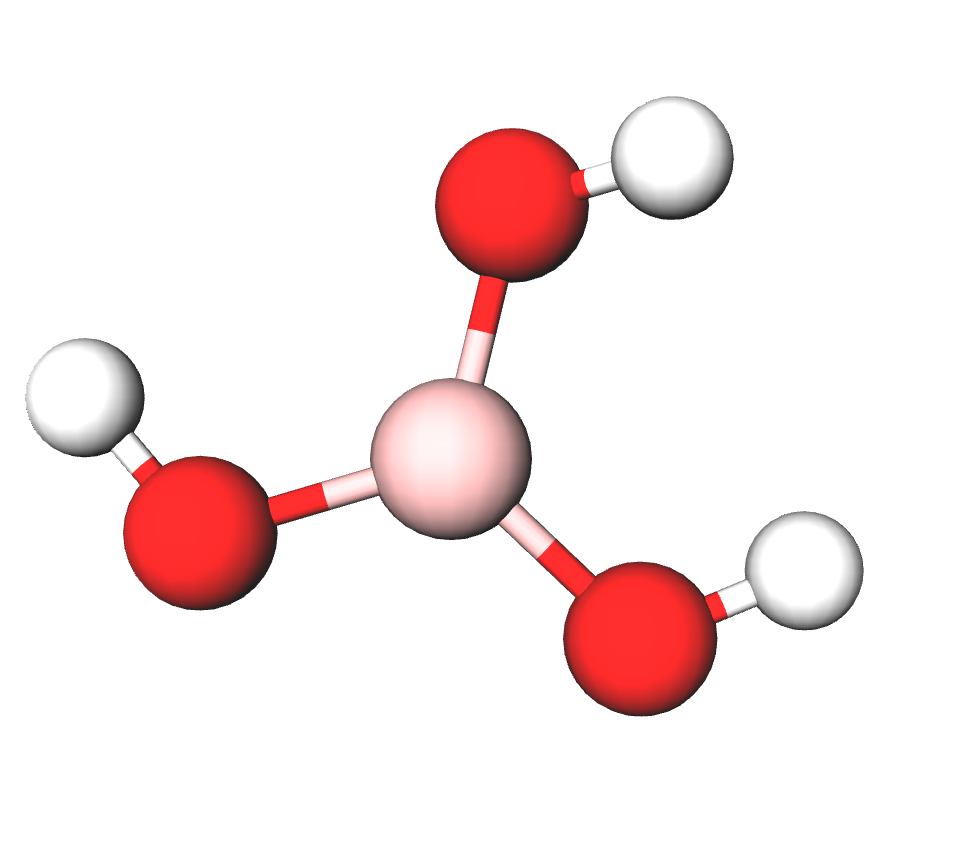 |
IUPAC | Boric acid |
INCI | BORIC ACID |
CAS | 10043-35-3 |
Molar mass | 61,83 g/mol |
Density | 1,435 g/cm3 |
Solubility | In water: 2,52 g/100 mL (0 °C) 4,72 g/100 mL (20 °C) 5,7 g/100 mL (25 °C) 19,10 g/100 mL (80 °C) 27,53 g/100 mL (100 °C) In alcohols, solubility increases, especially in glycerol. The solubility can reach up to 50g/100ml. |
Boric acid, also known as hydrogen borate, boric acid and orthoboric acid, is a weak, monobasic Lewis boric acid. However, some of the characteristics of boric acid in certain chemical reactions indicate that it is a tribasic acid. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber or as a raw material for other chemical compounds.
In metalworking, boric acid with borax is used as a welding flux. The boron ions facilitate the fusion of metals and prevent metal oxides from entering the alloy. In the jewellery industry, boric acid is used in combination with denatured ethyl alcohol to reduce surface oxidation and the appearance of stains on the surface of the metal in thermal processes (soldering, annealing).
In the wood industry, boric acid is used mixed with borax in a ratio of 4:5, which makes it very soluble in water, and this solution is used to protect wood from fire by impregnating it. In this way, the wood is impregnated with fire retardant. Boric acid protects and destroys existing wet and dry rot in wood. It can be used in combination with an ethyl glycol carrier to protect the exterior wood against fungi and insects. Concentrated borate-based compounds can be used to prevent the growth of slime, fungi or algae on the surface of wood, even in marine environments.
In water treatment boric acid is used as a pH buffer. Boric acid in equilibrium with its conjugate base (borate ions) is widely used (at concentrations ranging from 50 to 100 ppm boron equivalents) as a primary or supplementary pH buffer system in swimming pools.
In medicine, boric acid is used as an antiseptic for minor burns or wounds. A very dilute solution of boric acid is used as an eye wash. Dilute boric acid can be used as a vaginal wash to treat bacterial vaginosis due to excessive alkalinity, as well as candidiasis. As an antibacterial compound, boric acid can be used to treat acne. It is also used as a prevention of mycosis. When combined with alcohol, the resulting solution can be used to treat certain types of ear infection in humans and animals.
In animal husbandry, boric acid is used to treat hoof diseases in cattle. A solution is prepared which protects the hooves against fungal attack.
In horticulture boric acid is used as a fertiliser and mould protectant in crop and horticultural production. For example, tomatoes are irrigated heavily so that the soil substrate is kept moist. This becomes a breeding ground for moulds and fungi that weaken the growing tomatoes. When the substrate is fertilised with boric acid, the mould disappears and the boron ions are used by the plant as a trace element.
In nuclear power, boric acid is used as a neutron poison. The boron in boric acid reduces the likelihood of thermal decay by absorbing some thermal neutrons. Boric acid can be dissolved in spent fuel pools used to store spent fuel elements. The concentration is high enough to minimise neutron propagation. Boric acid was used in the aftermath of the Chernobyl accident, when boric acid was poured over reactor 4, which melted down, to prevent another reaction.
In pyrotechnics, boron is used to prevent the formation of amides in the reaction between aluminium and nitrates. A small proportion of boric acid is added to the composition to neutralise the alkaline amides which may react with aluminium. The boric acid is used as a coloring agent for the flame, coloring it green. Green flares and salutes contain boric acid.
In the lubricant industry, colloidal suspensions of boric acid nanoparticles dissolved in petroleum or vegetable oil can form excellent lubricants on ceramic or metal surfaces, with a coefficient of sliding friction that decreases with increasing pressure, from 0.10 to 0.02. Used to lubricate carrom and novuss game boards for faster play.
In the manufacture of insecticides, boric acid is used as an active ingredient in insecticides for the control of insects (cockroaches, termites, ants, flies, sugar gnats, etc.). It is recommended to spread boric acid in areas where insects congregate, or to prepare a saturated solution and to spray or smear it on surfaces where unwanted insects congregate.
In ceramics, orthoboric acid is used for the production of stamping compounds, fine silica-containing powders used in the lining of induction furnaces and in the manufacture of ceramics. One of the main industrial uses of boric acid is in the production of synthetic glass fibres. More commonly known as borosilicate or borosilicate glass.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
**- Trade in toxic substances shall be carried out in accordance with the provisions of the Law on the Control of Poisonous Substances of the Republic of Lithuania (current version). The purchaser must present a permit to work with toxic substances before purchasing toxic substances
Signal word: Danger |
Hazard icons:
|
Danger phrases: H360FD May impair fertility. May cause harm to the unborn child. |
Precautionary statements: P201 Obtain specific instructions before use. P202 Do not use unless all safety warnings have been read or understood. P281 Use required personal protective equipment. P308+P313 In case of exposure or anticipated exposure: seek medical advice. P405 Keep locked. |
00262002
Related products
(8 other products in the same category)












