AMMONIA (Ammonia water) 25%, L
8.00 €
CHEM002
Ammonia water, CAS 1336-21-6, ammonia, INCI AMMONIUM HYDROXIDE, ammonium hydroxide solution
Parameter | Attribute |
Ammonia water | Ammonia, ammonium hydroxide, ammonia solution, ammonium hydroxide solution |
Formula | NH4OH |
Structure | 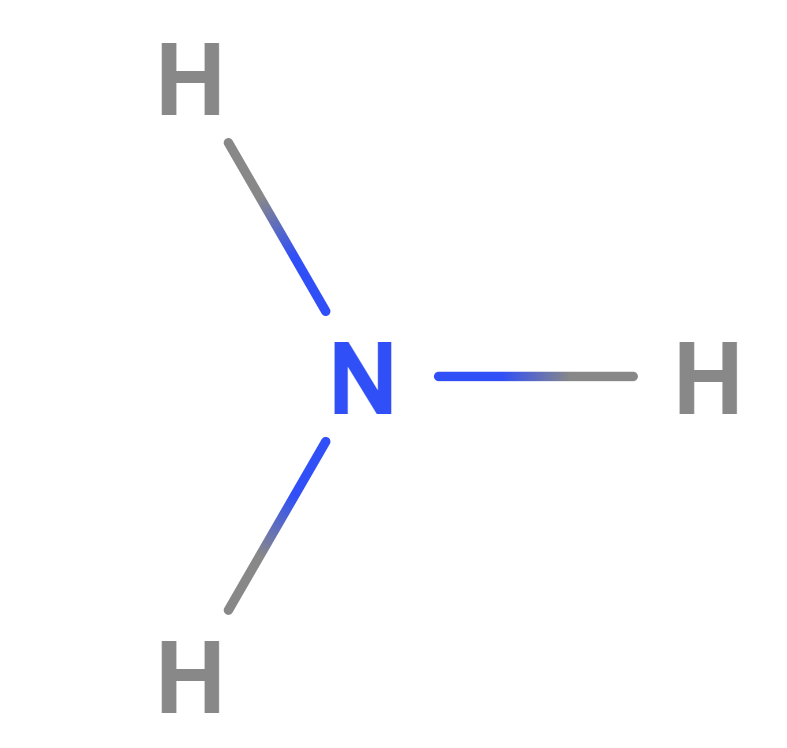 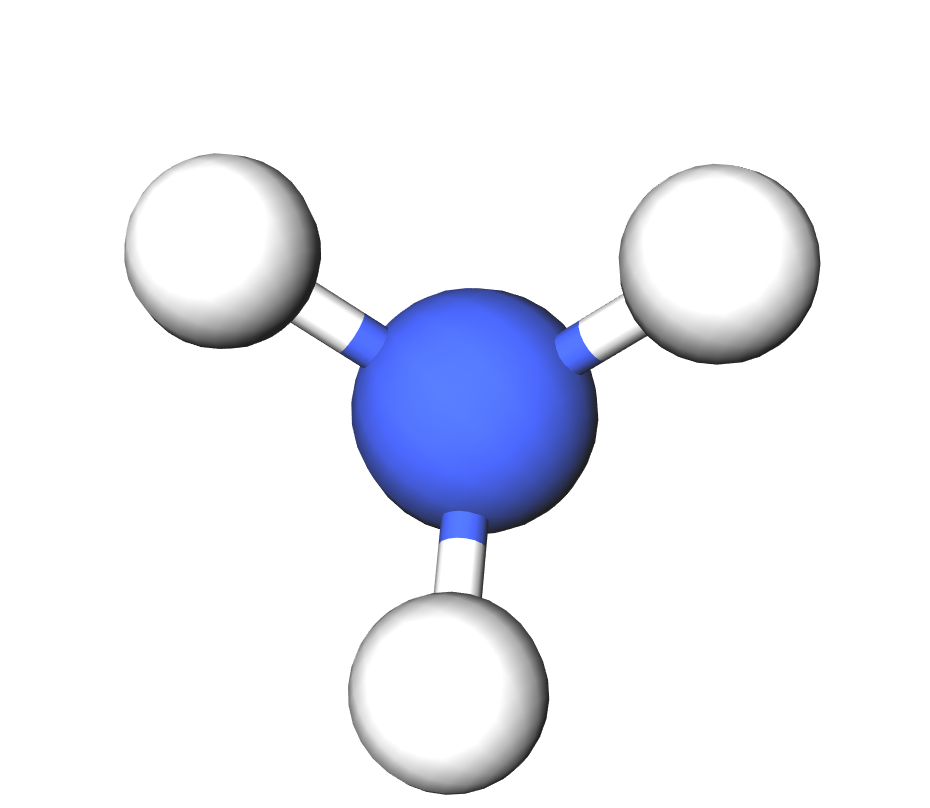 |
IUPAC | Ammonium hydroxidide |
INCI | AMMONIUM HYDROXIDE |
CAS | 1336-21-6 |
Molar mass | 35,04 g/mol |
Density | 0,91 g/cm3 (25 % w/w) 0,88 g/cm3 (35 % w/w) |
Solubility | 47% w/w (0 °C) 31% w/w (25 °C) 18% w/w (50 °C) |
NH3 is a gaseous compound of nitrogen and hydrogen. It is a colourless, poisonous, pungent-smelling gas that dissolves well in water (700 litres of NH3 gas dissolves in 1 litre of water). It is highly soluble in water (89,9 g/100 ml at 0 °C) and produces ammoniacal water (a weak alkaline, since ammonia reacts with water to form hydroxide ions). In this process, a small proportion of the ammonia molecules react with water. Ammonium and hydroxide ions are formed. At normal temperature and atmospheric pressure, ammonia is a gaseous substance. Ammonia gas is a pungent smelling, explosive, flammable and toxic gas.
In household whether in its pure form or as an ingredient in many household cleaning products, ammonia can be used to clean a wide range of household surfaces, from hot tubs, sinks and toilets to bathroom and kitchen worktops and tiles. Ammonia is also effective in removing household animal or vegetable grease soils or stains, such as cooking grease and wine stains. As ammonia evaporates quickly, it is commonly used in glass cleaning solutions to prevent streaks on glass.
In industry, aqueous ammonia is used to produce alkylamines. In the petroleum industry, ammonia is used to neutralise the acidic components of crude oil and to protect equipment from corrosion. In the mining industry, ammonia is used to extract metals such as copper, nickel and molybdenum from their ores. Ammonia is used in stack emission control systems, to neutralise sulfur oxides from the combustion of sulfur-containing fuels, as a method of controlling NOx, both catalytic and non-catalytic, and to increase the efficiency of electrostatic precipitators, and to control particulates. Ammonia is widely used in industrial refrigeration systems. The leather industry uses ammonia as a curing agent, as an abrasive and mould preventive in tanning solutions and as a protective storage agent for leather and fur. In the chemical industry, ammonia is widely used for the production of nitric acid and dyes. In pharmaceuticals, ammonia is used in the manufacture of sulfate preparations, vitamins and cosmetics. Ammonia is also used in the production of synthetic textile fibres (nylon, viscose (rayon), acrylic) and certain plastics (phenols, polyurethanes).
In water treatment. Ammonia is used to neutralise chloramine, which is used as a disinfectant. Chloramine is the preferred method of chlorination because of its ability to remain active for longer periods of time in still water pipes, reducing waterborne infection.
In food production, ammonia can be used as a baking powder. It is also used as an acidity regulator in the form of ammonia hydroxide. It is used in the beverage industry as a source of nitrogen needed by yeasts and micro-organisms.
In the furniture industry, ammonia water is used to treat wood containing stannous acid - for staining.
In agriculture, ammonia water (3%) is used to treat straw fed to cattle to increase its nutritional value. Aqueous ammonia solution can be used as a nitrogen fertiliser. Ammonia can also be used as an antifungal defoliant for cotton, as an antifungal agent in certain fruits and as a preservative for storing high moisture maize. Ammonia is also used in horticulture for the control/maintenance of weevils or moles.
In the jewellery industry, ammonia can be used to clean platinum, gold or silver jewellery.
In the laboratory, ammonia is used in traditional qualitative inorganic analysis, either as a complexing agent or as a base. It can be used for mild reduction of metals, e.g. in the silver mirror reaction.
In cosmetics, ammonia is used for hair coloring, most commonly ammonia acting as an alkaline agent. This allows the scales of the hair cuticle to be opened, which facilitates permanent coloring. Ammonia is an aqueous solution made from ammonia gas.
- pH adjuster: Stabilizes the pH of cosmetics
- Denaturant: Makes cosmetics unpleasant. Mainly added to cosmetics containing ethyl alcohol
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, colour, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H314 Severely burns the skin and damages the eyes. H335 May cause respiratory irritation. H412 Harmful to aquatic organisms, causes long-term effects. |
Precautionary statements: P260 Do not inhale vapours. P264 Wash hands thoroughly after use P280 Wear protective gloves/protective clothing/eye/face protection. P301+P330+P331 IN CASE OF DANGER: Rinse mouth. DO NOT induce vomiting P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Remove all contaminated clothing. Wash skin with water or shower P363 Wash contaminated clothing before putting it back on P304+P340 INFECTION: Remove the victim to fresh air; he/she must be in a comfortable position to breathe freely P310 Call the ACCIDENT CONTROL AND INFORMATION OFFICE/call a doctor immediately. P321 Special treatment (wash with 0.5% boric acid solution in case of contact with skin) P305+P351+P338 In case of contact with eyes: wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P405 Keep locked |
CHEM002
Related products
(8 other products in the same category)