CALCIUM CHLORIDE 95% (tech grade), kg
2.00 €
CHEM065
Calcium(II) chloride, CAS 10043-52-4, calcium dichloride, INCI CALCIUM CHLORIDE, for melting ice, for dusting roads, anhydrous calcium chloride
Parameter | Attribute | ||||
Calcium chloride | Calcium dichloride, calcium salt | ||||
Formula | CaCl2 | ||||
Structure | 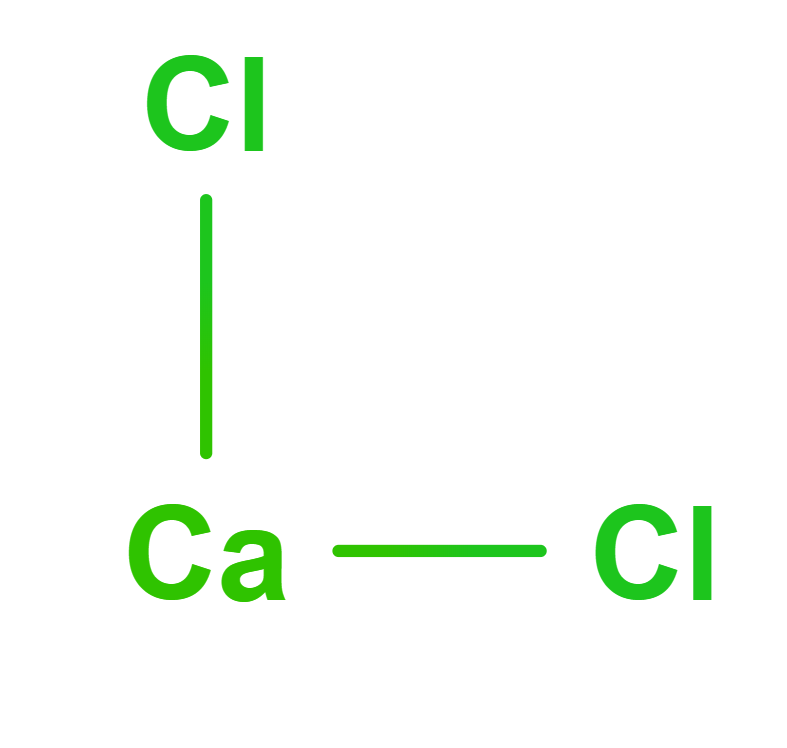 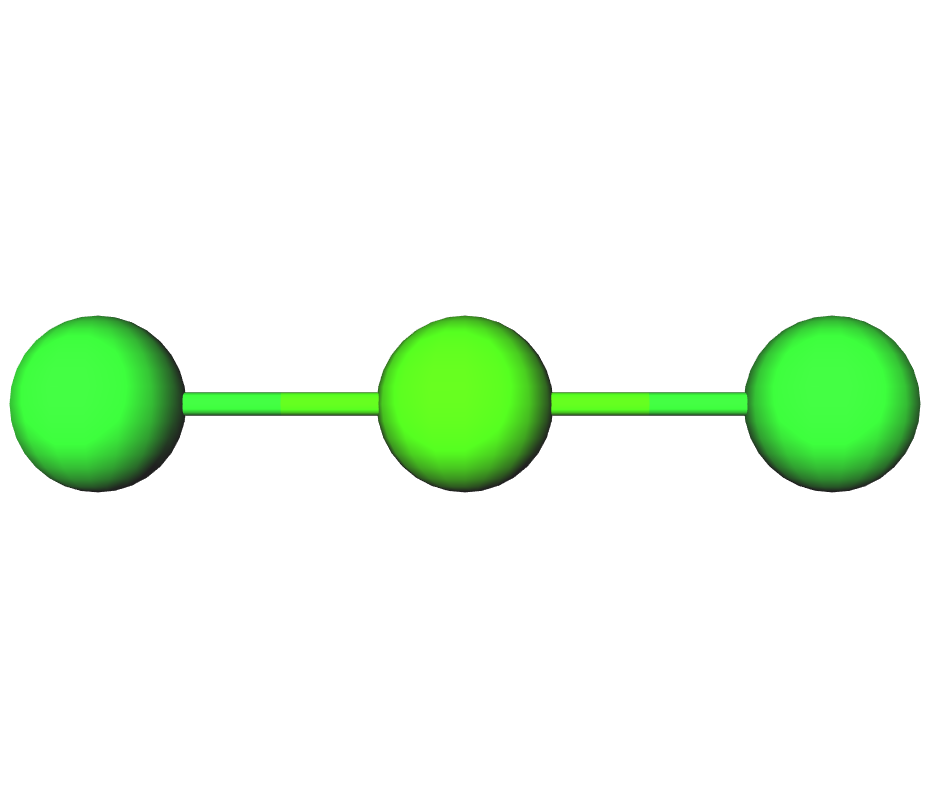 | ||||
IUPAC | Calcium chloride | ||||
INCI | CALCIUM CHLORIDE | ||||
CAS | 10043-52-4 | ||||
Molar mass | 110,98 g/mol | ||||
Density | 2.15 g/cm3 (Anhydrous) | ||||
Solubility | In water: Anhydrous: 74.5 g/100 mL (20 °C) | In ethanol: 18.3 g/100 g (0 °C) 25.8 g/100 g (20 °C) 35.3 g/100 g (40 °C) 56.2 g/100 g (70 °C) | In methanol: 21.8 g/100 g (0 °C) 29.2 g/100 g (20 °C) 38.5 g/100 g (40 °C) | In acetone: 0.1 g/kg (20 °C) | In phyridine: 16.6 g/kg |
Calcium chloride is an inorganic salt with the chemical formula CaCl2, a colourless crystalline solid that is very soluble in water at room temperature. Calcium chloride usually comes in a hydrated form with the formula CaCl2(H2O)x, where x denotes the number of water molecules, which can be 0, 1, 2, 4 and 6. These compounds are mainly used for icing and dust control. Since the anhydrous salt has hygroscopic properties, it is used as a drying agent.

On the road
In summer calcium chloride is used to reduce dust on shingle roads. Liquids are trapped on the road, which reduces the dustiness of the road. The concentrated solution retains a layer of liquid on dirty roads, which reduces dust formation. Fine dust particles are retained on the pavement, which provides a softening layer to the road surface. Without calcium chloride in the pavement, the particles would be able to move freely due to the low moisture content, which would lead to the formation of large dust aggregates that would disrupt the road surface. The use of calcium chloride in road construction reduces the need for layering by up to 50 % and the cost of replenishment by up to 80 %. Calcium chloride absorbs moisture and thus keeps the gravel road permanently wet, and binds the fine particles together, regardless of ambient temperature or humidity. There are different ways of using calcium chloride: powdered spreading, which absorbs moisture from the environment. The advantage of this method is that the effect of humidity is maintained for a longer period of time, the disadvantage is that the effect may not appear immediately. Calcium chloride can be dissolved in water and the available solution can be used to water the gravel road. The advantage of this method is that the effect is visible immediately, the disadvantage is that the persistence is shorter than with powder. Depending on the fractional composition of the gravel road, the yield is 100-150 kg of dry calcium chloride per 100 m of gravel road.
In winter, calcium chloride is used to stop ice formation/melt by lowering the freezing point of water. Calcium chloride freezes water down to - 52 °C instead of 0 °C. In this case, the advantage of using calcium chloride instead of normal salt is that ice can be melted even at lower temperatures, since sodium chloride melts ice down to - 9 °C, whereas calcium chloride is effective down to - 52 °C. Calcium chloride can be used either as a pure sprinkling agent or mixed with sodium chloride (salt). A solution can be prepared to give a significantly faster effect.
In the food industry it is used as a food additive (supplement). In the European Union, it is labelled with the E number E509. The average intake of calcium chloride in food supplements is thought to be 160-345 mg/day. It is considered by the US Food and Drug Administration to be a generally accepted and safe substance. It is used as a fortifying agent in the preservation of vegetables or fruit. Used as an electrolyte in sports drinks. Calcium chloride is also used in the production of extra salty pickles to avoid additional sodium in the diet. The freezing point reducing properties of calcium chloride are used to freeze caramel in caramel filled chocolates. It is sometimes used in brewing to replenish water with missing minerals. In cheese making, calcium chloride is sometimes added to processed (pasteurised/homogenised) milk to restore the natural balance between calcium and casein protein.
In aquaculture, calcium chloride can be used as a bioavailable source of calcium for shell animals (e.g. molluscs or dinoflagellates) whose shells are composed of calcium carbonate. Also it is used to make sea water mineralization.
In laboratories, calcium chloride can be used as a drying agent by adding it to drying tubes or cooling vessels.
In medicine, calcium chloride can be used in the treatment of magnesium poisoning, and in the treatment of internal hydrofluoric acid burns. It can be used to treat the rapid onset of calcium channel toxicity (blockage) caused by the side effects of other drugs such as diltiazem.
In research, calcium chloride in its aqueous form is used to effect genetic transformation of cells by increasing the permeability of the cell membrane, which promotes the uptake of DNA (allowing DNA fragments to easily enter the cell).
In the construction industry, calcium chloride is used in concrete mixes to speed up the initial curing (drying) process of concrete, but chloride ions cause corrosion of steel reinforcement and should not be used in reinforced concrete. Anhydrous calcium chloride can also be used for this purpose and can be used as a measure of the moisture content of concrete. It is also used as an admixture to improve the stiffness of concrete at low temperatures. It can be used for soil binding (when mixed with sodium silicate it hardens sandy soils). Calcium chloride is also used to increase the hardness of swimming pool water in order to reduce erosion of concrete in the pool.
Self-heating uses the exothermic water-solubility properties of calcium chloride, which can be applied in self-heating cans and heating mats.
In the paper industry, it is used to remove dyes from recycled paper, reducing dye losses.
In the production of activated carbon, it is used as a drying agent that absorbs moisture from the air. Due to its good solubility in water, it can be used as a mineral fertiliser for soils that need to be enriched with calcium or to reduce their acidity. On the surface, where it can be removed by skimming. Calcium chloride can also be used in wastewater treatment to remove various inorganic compounds (fluorides, silicates, phosphates, sulphates and various heavy metals) by binding them into insoluble salts which settle out and can be removed.
In blast furnaces, it is used as an additive to improve process characteristics.
In the petroleum industry, calcium chloride is used to increase the density of brines ('brines') that are free of solid impurities. It is also used to inhibit clay swelling in the aqueous phase of inverted emulsion drilling fluid (fluid).
In metals extraction, acts as a fluxing agent (lowering the melting point) for the industrial production of sodium metal by electrolysis from molten sodium chloride. Similarly, calcium chloride is used as a fluxing agent and electrolyte for the production of titanium, where it ensures the proper exchange of calcium and oxygen ions between electrodes.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Warning |
Hazard icons:
|
Danger phrases: H319 Causes severe eye irritation |
Precautionary statements: P262 Prevent contact with eyes, skin or clothing. P264 Wash eyes and hands thoroughly after use. P280 Wear protective gloves/protective clothing/use eye/face protection. P305 + P351 + P338 IN CASE OF CONTACT WITH EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P337 + P313 If eye irritation persists: seek medical advice. |
CHEM065
Related products
(8 other products in the same category)











