HYDRAZINE 4%, L
3.99 €
03170403
Stabilized hydrazine, CAS 10217-52-4, diazane, diamine, tetrahydridodiazane (N-N), diamidogen
Parameter | Feature |
Hydrazine hydrate | Stabilised hydrazine, diazane, diamine, tetrahydridodiazote (N-N), diamidogen |
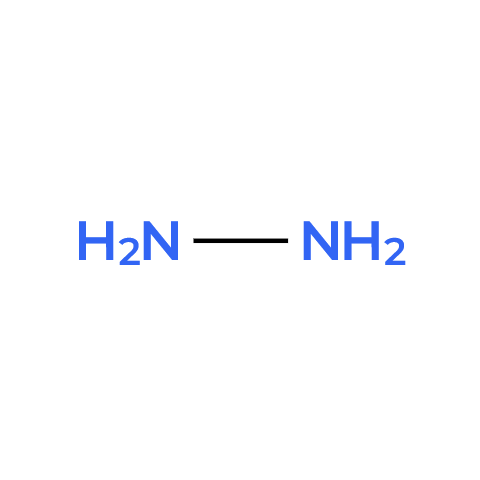
Formula | N2H4 |
Structure |
|
IUPAC | Diazan - diazane |
INCI | - |
CAS | 10217-52-4 |
Clay mass | 32.0452 g/mol |
Density | 1.021 g/cm3 (at 20 °C) |
Solubility | It is miscible in water in any proportion. Miscible with ethyl acetate, alcohols, hexanes, benzene, carbon tetrachloride, diethyl ether, trichloromethane and other polar solvents |
Hydrazine is an inorganic compound with the chemical formula N2H4. It is a colourless, flammable liquid with an odour similar to ammonia. Anhydrous hydrazine is very hazardous and is used commercially in solution as hydrazine hydrate (N2H4-H2O).
In the production of blowing agents, hydrazine is used as a precursor/raw material. The compounds produced are azodicarbonamide and azobisisobutyronitrile, one gram of which produces 100-200 ml of gas on decomposition. Azodicarbonamide is thermally decomposed into nitrogen, carbon dioxide, carbon monoxide and ammonia by passing through a liquid polymer precursor. These gases form bubbles in the liquid, which subsequently polymerises to form a light, foamy plastic. Hydrazine is also used as a blowing agent for the production of polymer (PE, PVC, PMMA, EVA, rubber, etc.) foams. In a similar way, hydrazine is reacted with sodium nitrite to produce sodium azide, a gas-releasing agent in automotive airbags.
In metalworking, hydrazine hydrate is used as a very strong reducing agent, i.e. it converts metals from an ionic (water-soluble) to a solid (metallic) state. This reaction is widely used in purification reactions of precious metals because it very easily removes all metals from solution.
In industrial plants,hydrazine is used as a corrosion inhibitor in industrial boilers, in nuclear/thermal power plant steam plants, and as an oxygen scavenger in ultra-high-pressure industrial plants. Hydrazine solutions from 1 to 4% are used. It is injected into electric water cooling systems to prevent corrosion and/or oxidation of pipes. This is achieved by capturing dissolved oxygen that would otherwise oxidise the metal. N2H4 + O2 → N2 + 2H2O. This reaction produces inert compounds: nitrogen gas and water. Hydrazine can also be used for metal conditioning/passivation when hematite is converted to magnetite: N2H4 + 6Fe2O3 → 4Fe3O4 + N2 + 2H2O. The same happens with copper oxides in non-ferrous metals: N2H4 + 4CuO → 2Cu2O + N2 + 2H2O
In agriculture, hydrazine is used in the production of active ingredients. Hydrazine is converted into heterocyclic rings such as pyrazoles and pyridazines e.g. cefazolin, rizatriptan, anastrozole, fluconazole, fluconazole, metazachlor, metamitron, metribuzin, paclobutrazole, diclobutrazole, propyconazole, hydrazine sulphate, diimidate, triadimefon, and diacylhydrazine insecticides. Hydrazine compounds are used as active ingredients in insecticides, miticides, nematicides, fungicides, antivirals, attractants, herbicides or plant growth regulators.
In the fuel industry, hydrazine decomposes into nitrogen and water in some types of hydrogen fuel cells and releases energy in an exothermic reaction. It actually produces slightly more energy than hydrogen, is easier to store because it is liquid at room temperature and does not require an expensive platinum catalyst. However, there are also higher production costs and the material is toxic.
Hydrazine (anhydrous) is used as rocket fuel in rockets. When mixed with the oxidising agent diazotetraoxide (N2O4), it produces a hypergolic mixture - so explosive that no ignition is required. As the fuel burns, three reactions take place to decompose hydrazine into ammonia, nitrogen and hydrogen gas. These highly exothermic reactions can cause the temperature in the reaction chamber to exceed 800 °C within a few milliseconds. Ammonia also decomposes, an endothermic process that removes some of the heat energy, but produces more nitrogen and hydrogen gases, which are pushed out of the rocket through the sealed nozzle and create attraction.
In leather and fabric processing, hydrazine is used to lighten the colours of leathers that are darkened by exposure to oxygen. It also helps to keep the reagents in the dye baths unchanged (by binding oxygen, reducing metal oxides to metals), so that all batches of fabrics are dyed evenly.
In wastewater treatment,hydrazine hydrate is used as a reducing agent to help remove harmful substances in wastewater such as heavy metal ions and nitrogen oxides. The strong reducing power of hydrazine hydrate makes it possible to break down these pollutants and reduce environmental damage. In addition, it can also be used to remove chlorine and chlorides from wastewater to further purify wastewater and improve water quality.
Important: add the product to the basket, fill in the recipient's details and confirm the order. Thank you!
To save your precious time, we will deliver your order to your address at your convenience !
*- The pictures of the goods may not correspond to the actual appearance, colour, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not be identical to the information on the product packaging. The information given on stock and prices may in some cases differ from the actual price and stock of the goods
**- Trade in toxic substances is carried out in accordance with the provisions of the Control of Poisonous Substances Act of the Republic of Lithuania (current version). The purchaser must present a permit to work with toxic substances before purchasing toxic substances
Signal word: DANGEROUS |
Danger icons:
|
Danger phrases: H301-Toxic by ingestion. H311-Toxic by contact with skin. H331-Toxic by inhalation. H317-May cause allergic skin reaction. H350-Can cause cancer. H410-Completely toxic to aquatic organisms, causes long-term effects. |
Precautionary statements: P280-Wear protective gloves/protective clothing/eye and face protection. P302+P352- In case of contact with skin: wash with plenty of water/. P304+P340-INSPIRATION: Get the person out in the fresh air and make sure he/she can breathe comfortably. P305+P351+P338-If contact with eyes: gently flush with water for several minutes. Remove contact lenses if present and easy to do so. Continue rinsing. P310-Call a poison control centre /doctor/ immediately. |
03170403
Related products
(8 other products in the same category)