COPPER SULFATE PENTAHYDRATE 98% (tech grade), kg
5.99 €
CHEM064
Blue vitriol, CAS 7758-99-8, copper blue, Blue vitriol (pentahydrate), INCI COPPER SULFATE, Bluestone (pentahydrate), Bonattite (trihydrate mineral).
Parameter | Attribute |
Copper sulfate pentahydrate | Cupric sulphate, Blue vitriol (pentahydrate), Bluestone (pentahydrate), Copper, Sulphate pentahydrate |
Formula | CuSO4 · 5H2O |
Structure | 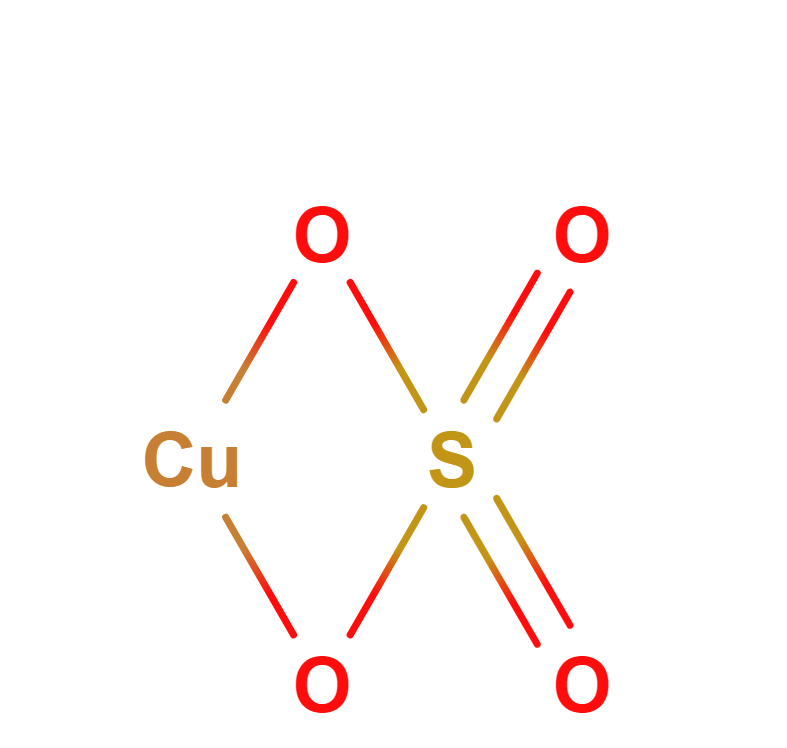 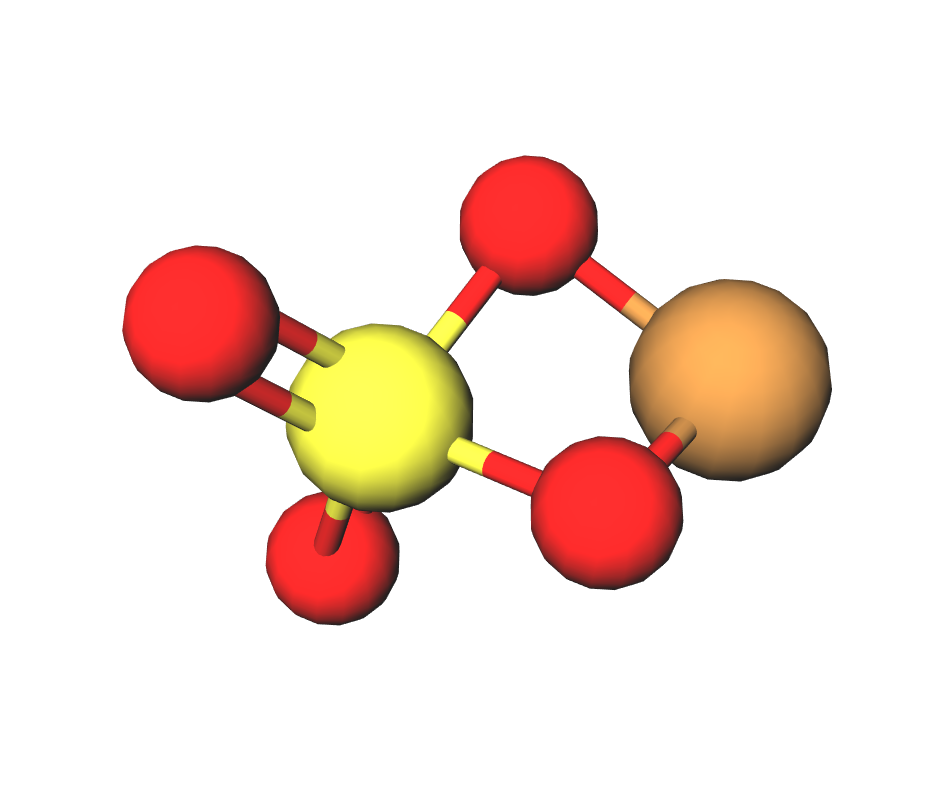 |
IUPAC | Copper sulfate pentahydrate |
INCI | COPPER SULFATE |
CAS | 7758-99-8 |
Molar mass | 249,68 g/mol |
Density | 2,3 g/cm3 |
Solubility | In water: pentahydrate |
Copper sulfate with the formula CuSO4∙5H2O, also known as bluestone because of the bright blue color of the crystals. It is easily soluble in water and has a wide range of applications. Copper sulfate is produced in accordance with GOST 19347-84 and is available in grades A first grade, B first grade and B second grade.
In terms of physico-chemical indicators, copper sulfate must comply with the following standards:
Name of indicator | Types | ||||
A | B | ||||
Highest | First | 1 type | 2 type | ||
1 | Percentage by weight of copper sulfate converted to CuSO4∙5H2O, not less than | 99,1 | 98,0 | 95,0 | 93,0 |
After conversion to copper, per cent | 25,20 | 24,94 | 24,17 | 23,67 | |
2 | Iron content by weight, %, not more than | 0,02 | 0,04 | 0,03 | 0,10 |
3 | Percentage by weight of free sulfuric acid, not more than | 0,25 | 0,25 | 0,25 | 0,30 |
4 | Percentage by mass of water insoluble precipitate, not more than | 0,03 | 0,05 | 0,04 | 0,10 |
5 | Mass fraction of arsenic, per cent, not more | 0,0002 | 0,012 | 0,012 | 0,03 |
Copper sulfate is used quite extensively, but some fungal species can adapt to elevated concentrations of copper ions. Mixing copper sulfate with calcium hydroxide (slaked lime) produces the Bordeux mixture, which consists of copper hydroxide and calcium sulfate, and is used for fungicide prophylaxis against grapes, melons and other berries.
Cheshunt compound is a mixture of copper sulfate and ammonium carbonate used in horticulture to prevent inhibition of seedling growth. Copper sulfate is not used in agriculture as an herbicide, but is excellent for the prevention of invasive aquatic plants or plants growing near water pipes. In swimming pools it is used to prevent algae.
In aquariums, copper sulfate is used to treat fish for parasitic infections or to kill aquarium snails. Copper ions are highly toxic to fish, so care should be taken when using in aquariums to avoid excessive amounts. Most algae can be killed with very low concentrations of copper sulfate. Copper sulfate inhibits the growth of some bacteria, e.g. Escherichia coli.
In horticulture, copper sulfate can be used in orchards for the prevention of major fungal diseases, the prevention of fungal diseases of fruit trees, berry plants, flowers and some vegetables, the correction of copper deficiencies in the soil and in fruit and berry plants, and the prevention of algae growth in flower pots; inhibition of moss and lichen growth on fruit trees, prevention of harmful organisms from intervening in wounds on fruit trees, impregnation of fruit wrapping paper to prevent the occurrence of rots during storage. Copper enhances the respiration rate of plants, resulting in improved protein metabolism and rapid plant development and growth. When copper is sufficient, plants become more resistant to diseases and pests, and the plants themselves inhibit the development of fungal diseases, resulting in a higher yield of fruits and seeds.
It is particularly useful to apply copper sulfate to peat soils (1-1,5 g of copper sulfate per m2 of soil). This amount of fertiliser is usually sufficient for 5-6 years. It is also advisable to spray a solution of copper sulfate on plants during the growing season: dissolve 2-5 g of copper sulfate in 10 litres of water.
Before sowing, it is recommended to soak the vegetable seeds in a solution of copper sulfate: 2 g of copper sulfate in 1 litre of water. Copper sulfate is generally poorly soluble in cold water and should only be dissolved in hot water. It is advisable to spray fruit trees and shrubs with copper sulfate solution in early spring, before the buds have burst, and in late autumn, after the plants have shed their leaves.
A 1 % aqueous solution is usually prepared. Preparation of a 10 l solution: dissolve 100 g of copper sulfate in 10 l of water. Spray the plants in spring before bud swelling. When planting fruit trees, soak the roots in a copper sulfate solution to prevent root tubers. Warning! Do not use very strong concentrations of solutions, they may burn the plants! It is recommended to treat plants with copper sulfate to prevent the following symptoms: chlorosis (discolouration of leaves, shoots, stems), deformation, spotting, leaf drop, wilting, necrosis (death of tissues or plant parts, blackening), etc.
- For the control of aphids and lichens, prepare a solution of 50 g/1L;
- Solution for the eradication of sulfur rot, powdery mildew - 30 g/L,
- For protection against fungus - 100 g of copper sulfate mixed with 5 litres of water + 100g of slaked lime mixed with 5 litres of water. The substances are mixed separately and when fully dissolved are mixed together. Duration of action about 5 hours.
Copper sulfate is used as a fertiliser:
- Increases pollen viability
- Promotes the formation of reproductive organs and the accumulation of sugars
- Reduces crop wilting
- Increases plant resistance to fungal and bacterial diseases
- Essential when applying high nitrogen rates, especially with ammonium fertilisers
For cereals: 50-100 g/ha
Rapeseed: 50-100 g/ha
Maize: 50-100 g/ha
Vegetables: 50-100 g/ha
In construction industry, copper sulfate is used as a concrete admixture to increase water absorption and to provide disinfectant properties against moulds, mildew and slime. It can also be used to remove salt deposits and rust from clinker, brick, plaster or concrete surfaces. Copper sulfate is used to impregnate wood to protect it against wood fungus, mould and decay. It is easy to use because its bright color makes the coated surface visible. Copper sulfate can also be used in the manufacture of mineral paints because of the color it gives, which can be blue, green or brown.
In veterinary medicine, copper sulfate is used for the prevention and treatment of hoof diseases by preparing copper sulfate solution baths for animals. It is also used in feed additives and premixes as a source of copper. Concentrations used range up to 0,2-0,3% by weight of the premix.
In school experiments, copper sulfate is used to grow crystals. A saturated solution is prepared by heating the water and dissolving the copper sulfate in it with stirring until it no longer dissolves. When the crystals appear on the thread, remove it from the solution and select the largest and smoothest crystal.Tie the crystal to the new thread so that it is in the centre of the vessel. Keep growing until you have a nice, big crystal. Remember to add more solution from time to time as the liquid slowly evaporates. To do this, prepare the same solution in another jar and leave it to stand.


Copper sulfate can also be used to color the flame. As the flame burns, the copper ions color it bright green. Alcohols, such as methanol or ethanol, are commonly used as a fire source.
In the metalworking industry, copper sulfate is used as a component of electrolytic plating baths. Metal plating is usually a multi-layer process: the first layer is oxidised iron (black in color). This layer has a strong adhesion to the surface of the iron and has a porous structure, which improves the adhesion of other layers. The second layer is applied to the copper by reduction of copper sulfate, which fills the pores and acts as a 'bonding/amortising' material between the iron and the third layer, i.e. nickel or chromium.
Copper sulfate is also used in copper or zinc plate etching processes due to its mild etching properties. It can also be used in jewellery to etch drawings. This technique is known as the "Champagne" technique.
In dyeing, copper sulfate is used as a dye fixative in vegetable dyeing. It usually brings out the green color and/or green tones in the paint. Very often paints containing copper sulfate are found in the decoration of metals and ceramics. A possible use of copper sulfate in art is the coating of surfaces with a solution of copper sulfate and the drying of the solution to form crystals which form special shapes on the surfaces. In printing, copper sulfate is used as an additive in inks and glues to protect books from insect attack.
In the chemical industry the use is rather limited. It is most commonly found in the production of cellulose acetate fibres. Anhydrous copper sulfate is used as a dehydrating agent to form and modify the positions of carboxy (acetate) groups in compounds. Copper sulfate pentahydrate mixed with potassium permanganate is used to produce primary alcohols.
In the analysis copper sulfate is used as an auxiliary. Copper sulfate is used in blood tests to detect anaemia. When blood is added to a solution of copper sulfate at a known density, drowning of the blood droplet is observed. If the droplet does not sink or sinks very slowly, the blood is deficient in haemoglobin. If the blood droplet sinks quickly, this indicates that there is sufficient haemoglobin in the blood. Copper sulfate is used to make Felling's and Benedict's solutions which reduce sugars. During sugar reduction, the soluble blue copper(II) sulfate is converted to insoluble copper(I) oxide with a red color. Copper sulfate is also used to make Biuret reagent, which is used for protein analysis.
In medicine, copper sulfate has been used in antiemetic preparations. It is now found to be too toxic to be used for this purpose due to its high concentration, although it is classified by the World Health Organisation as an antidote.
In the food industry it is registered as food additive E519 (Artificial acidity regulator, essential mineral, preservative).
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H350 May cause cancer. H360D May cause harm to the unborn child. H302 Harmful if swallowed. H318 Severely damaging to eyes. H317 May cause allergic skin reaction. H373 May cause damage to organs by prolonged or repeated exposure. H410 Very toxic to aquatic organisms, causing long-term effects. |
Precautionary statements: P201 Obtain specific instructions before use. P273 Keep out of the environment. P280 Wear protective gloves/protective clothing/use eye/face protection P301+P312 CAUTION: If you feel unwell, call the Poison Control and Information Bureau or seek medical attention. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P302 + P352 IN CONTACT WITH SKIN: Wash with plenty of soap and water. P308 + P313 In case of contact or suspected contact: seek medical advice. |
CHEM064
Related products
(8 other products in the same category)














