SODIUM TETRABORATE DECAHYDRATE (Borax), 99%, kg
124.99 €
02112008
Borax, CAS 1303-96-4, Sodium borate, INCI SODIUM BORATE, Sodium tetraborate, Disodium tetraborate, E285, Tétraborate de sodium.
Parameter | Attribute |
Sodium tetraborate decahydrate | Borax, sodium borate, sodium tetraborate, disodium tetraborate, E285, Tétraborate de sodium |
Formula | Na2B4O7·10H2O, |
Structure |  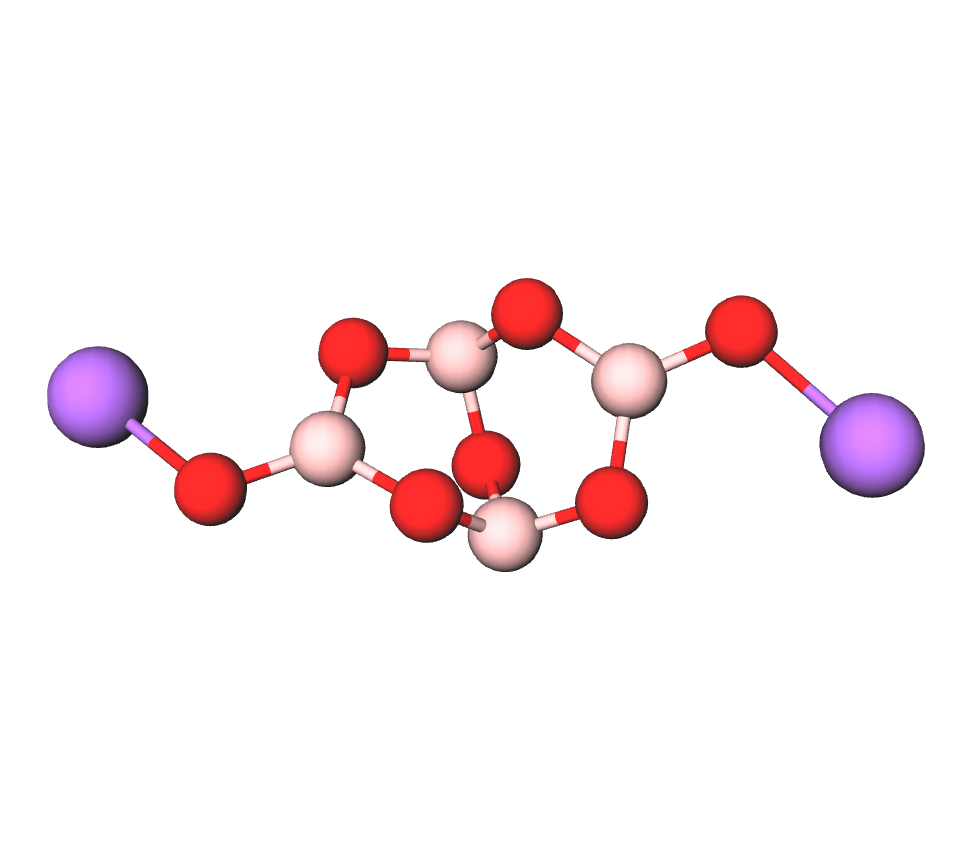 |
IUPAC | Sodium tetraborate decahydrate |
INCI | SODIUM BORATE |
CAS | 1303-96-4 |
Molar mass | 381,37 g/mol |
Density | 1,73 g/cm3 |
Solubility | In water 31,7 g/l, very soluble in glycerol |
Borax, also known as sodium borate, sodium tetraborate or disodium tetraborate, is an important boron compound, mineral and salt of boric acid. Powdered borax is white, consisting of soft colorless crystals which dissolve in water.
In metalworking, borax is used as a welding flux. A mixture of borax and ammonium chloride is used as a flux for welding iron and steel. This lowers the melting point of unwanted iron oxide (precipitate) and allows it to escape. Borax is also used mixed with water as a flux in the soldering of jewelry metals such as gold or silver, where the molten solder allows the surface of the metal to be wetted and to flow evenly into the joint. Borax is also a good flux in the melting of tungsten with zinc, resulting in soft soldering of tungsten. Borax is often used as a flux when welding metals by contact welding. Borax has replaced mercury in gold recovery because, unlike mercury (which relies on gold being combined and separated from impurities), this method relies on the ability of borax to lower the melting point of all minerals. Since gold is generally the heaviest of these minerals, borax allows gold to be concentrated at the bottom of the crucible. The process requires considerably less heat than conventional purification methods, which can be obtained even in remote locations (using coal). Once the ore has been crushed into a fine powder, it is lightly roasted so that only the heaviest minerals remain in the pan. The mineral mixture is then thoroughly mixed in a 3:1 (by volume) ratio (three parts minerals to one part borax) of borax and a few drops of water. This mixture is then heated until the entire mixture is melted, after which time the molten gold droplets accumulate on the bottom of the crucible.
In the wood industry, boric acid is used in a 4:5 mixture with borax, which makes it very soluble in water, and this solution is used to protect wood from fire by impregnating it. In this way, the wood is impregnated with fire retardant. Borax prevents and destroys existing wet and dry rot in wood. It can be used in combination with an ethyl glycol carrier to protect the exterior wood against fungi and insects. Concentrated borate-based compounds can be used to prevent the growth of slime, fungi or algae on the surface of wood, even in marine environments. Borax formulations are used to prevent rotting in the manufacture of classic wooden boats.
In water improvement borax is used as a pH buffer. Borax is widely used (at concentrations ranging from 50 to 100 ppm boron equivalents) as a main or additional pH buffer in swimming pools. Borax alone does not have a significant effect on hardness cations, although it has been used for water softening. Its chemical equation for water softening is given below:
Ca2(aq) + Na2B4O7(aq) → CaB4O7(k)↓ + 2Na(aq)
Mg2(aq) + Na2B4O7(aq) → MgB4O7(k)↓ + 2Na(aq)
The introduction of sodium ions does not produce 'hard' water. This method is suitable for removing temporary and permanent types of hardness. Water softened in this way is used for technical applications.
In medicine, borax is used as an antiseptic to treat minor burns or wounds. A highly diluted solution of borax is used as an eye wash or as a storage liquid for eye lenses. A dilute solution of borax can be used as a vaginal wash to treat bacterial vaginosis due to excessive alkalinity as well as candidiasis. As an antibacterial compound, borax can be used to treat acne. It is also used as a prevention of mycosis. When combined with alcohol, the resulting solution can be used to treat certain types of ear infections in humans and animals. Borax is used as a treatment agent for snakeskin disease.
In livestock farming, borax is used to treat hoof diseases in cattle. A solution is prepared to protect the hooves against fungal attack.
In crop and horticulture, borax is used as a micronutrient fertilizer and mold protection. For example, tomatoes are irrigated heavily so that the soil substrate is kept moist. This becomes a breeding ground for molds and fungi that weaken the growing tomato. When the substrate is fertilized with borax, the mold disappears and the boron ions are used by the plant as a trace element. Borax is also used to enrich beet plants with boron when their leaves begin to wilt.
In nuclear power, borax is used as a neutron poison. Boron reduces the likelihood of thermal decay by absorbing some thermal neutrons. Borax can be dissolved in spent fuel pools used to store spent fuel elements. The concentration is high enough to minimize neutron propagation. Borax was used in the aftermath of the Chernobyl accident - borax was poured over reactor 4, which had melted down, to prevent another reaction.
In pyrotechnics, borax is used as a coloring agent for the flames, coloring them green. Green flares and salutes contain borax.
In insecticides, borax is used as an active ingredient in the manufacture of insecticides for the control of insects (cockroaches, termites, ants, flies, sugar gnats, etc.). It is recommended to spread borax in areas where insects congregate, or to prepare a saturated solution and to spray or smear it on surfaces where unwanted insects congregate.
In ceramics, borax is used for the production of stamping compounds, fine silica-containing powders used in the lining of induction furnaces and for ceramics. It is also used in enamels to lower the melting point and to spread it more evenly over the surface.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H360FD May impair fertility. May cause harm to the unborn child. H319 Causes severe eye irritation. |
Precautionary statements: P201 Obtain specific instructions before use. P202 Do not use unless all safety warnings have been read or understood. P280 Wear protective gloves/protective clothing/eye/face protection. P305+P351+P338 IN CASE OF CONTACT WITH EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P308+P313 In case of exposure or if contact is anticipated: seek medical advice. P405 Keep locked. |
02112008
Related products
(8 other products in the same category)













