POTASSIUM PERMANGANATE 99%, kg
4.50 €
01191301
Potassium permanganate, CAS 7722-64-7, Chameleon mineral, Condy’s crystals, Potash permanganate, Hypermangan.
Parameter | Attribute | |
Potassium permanganate | Potassium permanganate, Chameleon mineral, Condi crystals, Potash permanganate, Hypermanganese | |
Formula | KMnO4 | |
Structure | 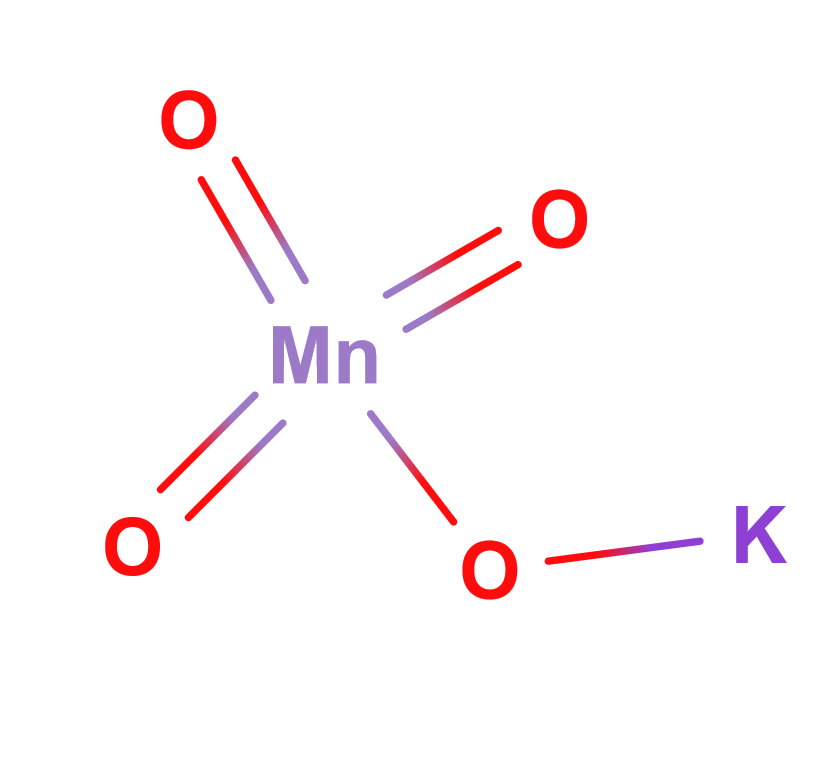  | |
IUPAC | Potassium manganate(VII) | |
CAS | 7722-64-7 | |
Molar mass | 158,034 g/mol | |
Density | 2,70 g/cm3 | |
Solubility | In water: 76 g/L (25 °C) 250 g/L (65 °C) | Decomposes in alcohols and organic solvents |
Potassium permanganate is an inorganic compound with the chemical formula KMnO4 and consisting of K+ and MnO-4. It is a purple-black crystalline salt which dissolves in water, to give an intensely reddish or purple solution.
In agriculture, potassium permanganate is used as an organic nitrogen liberator in soil. By oxidizing ammonium and nitrite ions to nitrate, potassium permanganate converts nitrogen more readily available to plants.
In horticulture, potassium permanganate is used as an effective plant disinfectant and as an active source of oxygen, thus protecting plants from pests and improving oxygen availability in the soil. At the same time, potassium permanganate serves as a potassium source. Through these mechanisms it accelerates plant growth. Potassium permanganate shall not exceed 0,001 %. Low potassium permanganate concentrations can help to keep fruit and vegetables ripe for longer. Wash ripe fruit and vegetables with a solution of this concentration, and this will help you keep them fresh for longer, a solution of 0.1-0.15% (per liter) 1-1.5 g of potassium permanganate in a liter of water) will help disinfect seeds or bulbs. Leave the seeds or bulbs in the prepared solution for about two hours, drain, the seedling root disease called blackleg or dieback, is often a real headache for gardeners. These plant diseases can be quite simple to treat - just water the affected plants with 0,05 Permanganate (dissolve 0.5 g of potassium permanganate in a liter of water). If your plants are attacked by powdery mildew, a combination of potassium permanganate and a solution of calcined soda: dissolve 50 g of calcined soda in 10 liters of water 10 g of potassium permanganate. Spray the affected plants with this solution. If you have fungi in your garden that you want to eradicate, 0.25% potassium will help. permanganate solution (dissolve 2,5 g of potassium permanganate in a liter of water). Use this solution to water the areas where unwanted fungi have grown – watering at least several times. Too frequent watering can cause indoor plants to flies. It is recommended that the plant's soil be amended and its pot washed with a high concentration of potassium permanganate solution. If the soil is damaged by pesticides or other chemical contaminants, it can be treated with potassium permanganate solution, which should be injected into the damaged areas of the soil. A 2-4 per cent potassium permanganate solution is recommended (10 liters of 20-40 g of potassium permanganate in 10 liters of water). This solution will effectively neutralize harmful substances and improves the condition of the soil.
In aquaculture and aquaria, potassium permanganate is used as an effective treatment for fish against parasites. It is used both in closed aquaculture farms and in aquariums and wild fish farming. In aquariums, it is used to treat both freshwater and marine aquatic fish.
In the film industry, potassium permanganate is one of the main chemicals used in film and television industry to 'age' sets and fabrics. Prepared potassium permanganate solution produces a brown MnO2 and creates a 'hundred-year-old' or 'antique' look on Hessian fabric (cloth), ropes, wood and glass.
In the ignition of a fire potassium permanganate is added to 'plastic sphere dividers' to form the backs of fires, burnouts and controlled ignitions. Polymer spheres similar to ping-pong balls containing small amounts of permanganate, injected with ethylene glycol and projected towards the desired location to be ignited, and then spontaneously ignites. Both hand-held and helicopter or boat-mounted plastic balls.
In the food industry, ethylene absorbers extend the shelf life of bananas even at high temperatures. See this this effect can be exploited by packaging bananas in polyethylene with potassium permanganate. By removing ethylene through oxidation, permanganate delays the ripening, increasing the shelf life of the fruit to 4 weeks without refrigeration.
In water treatment, potassium permanganate is widely used in the water treatment industry. It is used as a regenerative chemical for iron and hydrogen sulfide (rotten odor) from borehole/well water. Potassium permanganate is dissolved in water and the solution is dosed into water clarification filters. It is used in the presence of extremely high iron and organic matter levels, when no removal by conventional aeration. Historically, it has been used for disinfection of drinking water and can turn water pink. It is currently used to control harmful organisms such as zebra mussels, in freshwater collection and treatment systems.
In organic synthesis, the main use of KMnO4 is as a reagent for the synthesis of organic compounds. It is used in large quantities in the production of ascorbic acid, chloramphenicol, saccharin, isonicotinic acid and pyrazinic acid.
In analysis and school experiments, potassium permanganate can be used to quantify the amount of oxidizable organics in aqueous samples. The value found is known as the permanganate value. In analytical chemistry, a standardized aqueous solution of KMnO4 is sometimes used as an oxidizing titre for redox titration (permanganometry). When potassium permanganate is titrated, the solution turns a light purple hue, which darkens when excess titrant is added to the solution. In a related way, it is used as a reagent for the determination of the Kappa number of wood pulp. Oxalic acid reduction is often used to standardize KMnO4 solutions. KMnO4 is used for qualitative organic analysis to investigate the presence of unsaturated compounds. Reaction with double or triple bonds (-C = C- or -C≡C-) results in a color fade from reddish pink to brown. A positive test is also found with aldehydes and formic acid (and formiates). Aqueous, acidic solutions of KMnO4 are used to collect gaseous mercury in exhaust gases for stationary source emissions testing. Potassium permanganate is sometimes included in survival kits: as a hypergolic starter (mixed with glycerol antifreeze from a car radiator) as a water sterilizer - and on distress signals on snow. A drop of glycerin on finely crushed potassium permanganate will ignite a flame within seconds. When dry potassium permanganate is heated, it decomposes and releases oxygen, which can be used in other experiments.
In animal husbandry, potassium permanganate is used as a treatment and prophylaxis for hoof diseases. It can also be used for the treatment and prevention of horn and hoof fungus. It can be used as a veterinary preparation for the disinfection of animal wounds. Due to its non-toxicity and effectiveness, it is used on organic farms.
In medicine, potassium permanganate is used to treat many skin diseases. These include fungal foot infections, impetigo, pemphigus, superficial wounds, dermatitis and tropical ulcers. It is on the World Health Organization’s list of essential medicines, the safest and most effective medicines needed in the health system.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
**- Implementing Regulation (EU) 2019/1148 of the European Parliament and of the Council of 20 June 2019 on trade in and use of explosive precursors, amending Regulation (EC) No 1907/2006 and repealing Regulation (EU) No 98/2013 (OJ L 186, 11.7.2019, p. 1). The product shall only be sold to Legal Persons, farmers or self-employed persons after a signed confirmation of the authorization to purchase and of the legitimate use by completing a declaration form.
Signal word: Danger |
Hazard icons:
|
Danger phrases: H272 May increase the risk of fire, oxidizer H302 Harmful if swallowed H314 Severely burns skin and damages eyes H361d Suspected of causing harm to the unborn child H373 May cause damage to organs (brain) by prolonged or repeated exposure (inhalation) H410 Very toxic to aquatic organisms, causes long-term effects |
Precautionary statements: P220 Keep away from clothing and other combustible materials P273 Keep out of the environment P280 Wear protective gloves/eye protection |
01191301
Related products
(8 other products in the same category)















