AMMONIUM NITRATE, 98%, kg
3.00 €
Ammonium nitrate, CAS 6484-52-2, smoky nitrate, INCI AMMONIUM NITRATE
Parameter | Attribute |
Ammonium nitrate | Ammonium nitrate, fuming nitrate |
Formula | NH4NO3 |
Structure |  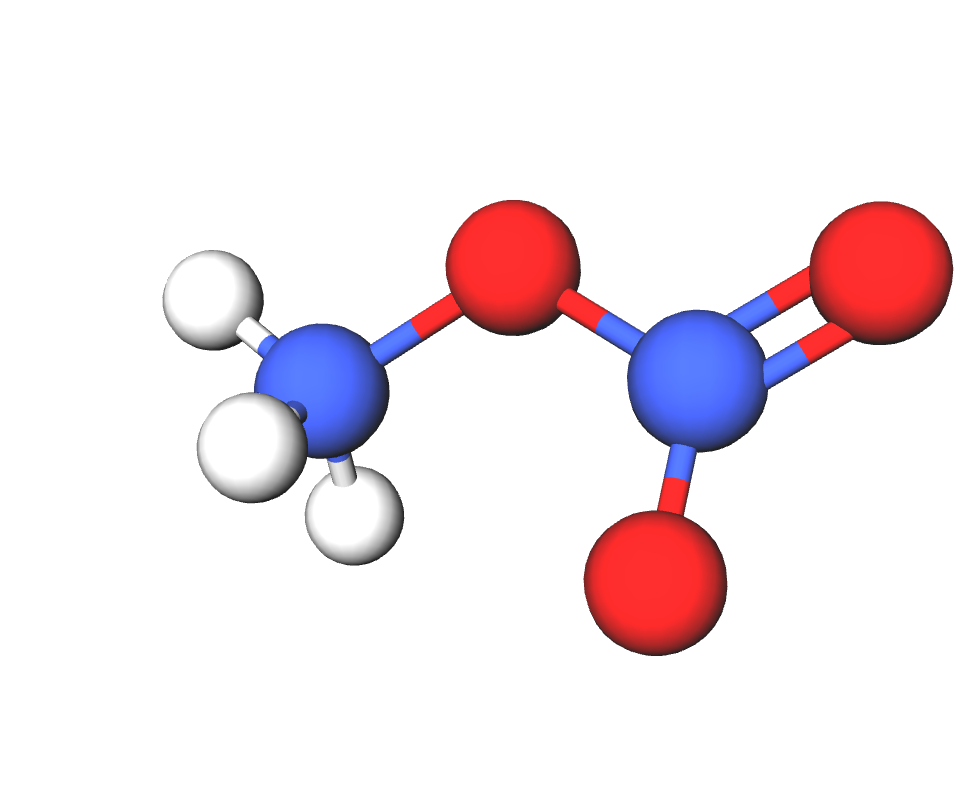 |
IUPAC | Ammonium nitrate |
INCI | AMMONIUM NITRATE |
CAS | 6484-52-2 |
Molar mass | 80,043 g/mol |
Density | 1,725g/cm3 (crystal) |
Solubility | In water, endothermic: 118 g/100 ml (0 °C) |
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt composed of ammonium and nitrate ions. It is very soluble in water and hygroscopic as a solid, although it does not form hydrates.
In the refrigeration industry, ammonium nitrate can be used in some rapid chilling solutions because its solubility in water is strongly endothermic (heat-absorbing, or freezing). It can be used as a refrigerant in off-grid refrigeration systems or to maintain critical refrigeration systems in emergency operation. This method is relatively short term and would require very large quantities of ammonium nitrate for constant cooling, which is not economically viable.
In the cleaning industry, ammonium nitrate is used as a safe bleaching agent because of its bleaching properties, but it is much more stable than peroxides.
In horticulture, gardening and agriculture, ammonium nitrate, more commonly known as ammonium nitrate, provides the plant with the necessary amount of nitrogen, which is particularly important during the period of intense plant growth. Fertilization quickly eliminates the signs of 'starvation' in the plant, resulting in faster root development, faster nutrient supply, faster growth and development, and less yellowing of the leaves. Nitrogen stimulates and regulates many of the plant's vital and growth-related processes. Ammonium nitrate fertilization reduces water consumption, makes plants more protein-rich and sugary, and prolongs their growing season. It is one of the most concentrated nitrogen fertilizers. Due to its good solubility, it produces a fast fertilization result. The NPK value is 34-0-0 (34 % nitrogen). It is less concentrated than urea (46-0-0), which makes transporting ammonium nitrate slightly less sensible (it requires more). The advantage of ammonium nitrate over urea is that it is more stable and does not rapidly lose nitrogen to the atmosphere during storage and transport. Composition: Total nitrogen (N) content 34,4 % by weight (nitrate nitrogen content 17,2 % by weight and ammonia nitrogen content 17,2 % by weight). Recommended application rate: 20-30 g/m²
In laboratories and schools, ammonium nitrate is used to achieve low temperatures in cooling baths. Temperatures as low as -160C can be achieved by dissolving pure ammonium nitrate and even lower temperatures by mixing with other salts. This is very useful for condensing or cooling operations without a water source. This process can also be used to achieve faster crystal growth effects.
In pyrotechnics, ammonium nitrate is used as a component of the smoke curtain. Since ammonium nitrate has more oxygen than it consumes, it is mixed with other materials that are oxygen deficient, such as sugar, flour, paper, cotton wool or other types of carbohydrates. The combination of the products produces a thick white smoke curtain with a characteristic odor. In the case of incompatible ratios, smoke formation is very slow or open flame combustion is initiated, which in turn reduces the smokiness.
In cosmetic manufacturing, ammonium nitrate is used as a buffer to maintain a uniform pH throughout the life of the product.
- pH adjuster: Stabilizes the pH of cosmetics
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Warning |
Hazard icons:
|
Danger phrases: H272 May increase the risk of fire, oxidizer H319 Causes severe eye irritation. |
Precautionary statements: P210 Keep away from heat sources, hot surfaces, sparks, naked flames or other sources of ignition. Do not smoke. P220 Keep away from flammable materials. P221 Take all precautions to avoid mixing with combustible materials. P264 Wash hands thoroughly after use P280 Wear protective gloves/protective clothing/eye/face protection. P305 + P351 + P338 IN CASE OF CONTACT WITH EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P370+P378 In case of fire: use water to extinguish |
Related products
(8 other products in the same category)












