HYDROCHLORIC ACID 28-36%, L
3.80 €
Muriatic acid, CAS 7647-01-0, hydrogen chloride, INCI HYDROCHLORIC ACID.
Parameter | Attribute | |||
Hydrochloric acid | Muriatic acid, hydrogen chloride, salt acid | |||
Formula | HCl | |||
Structure | 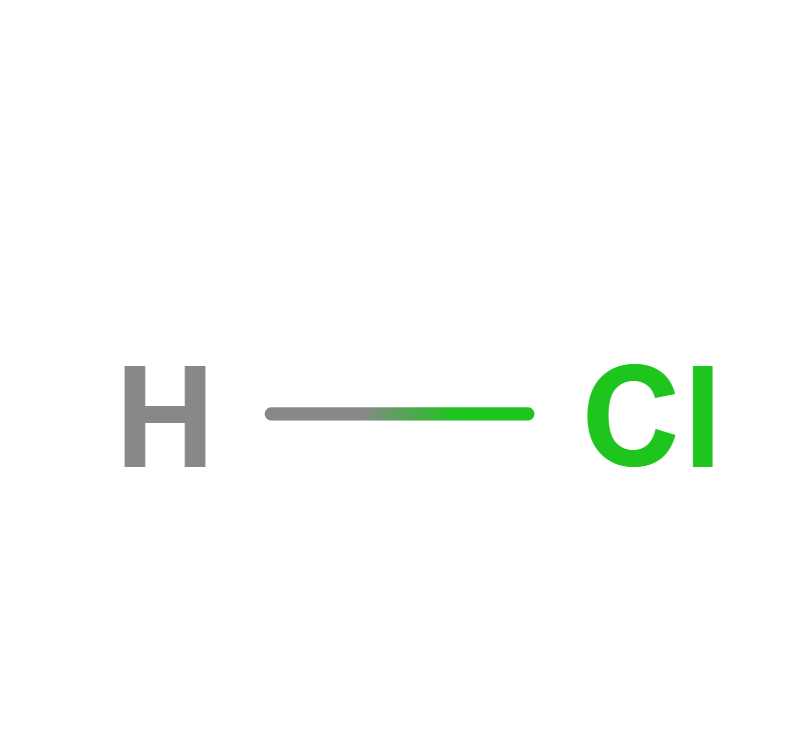 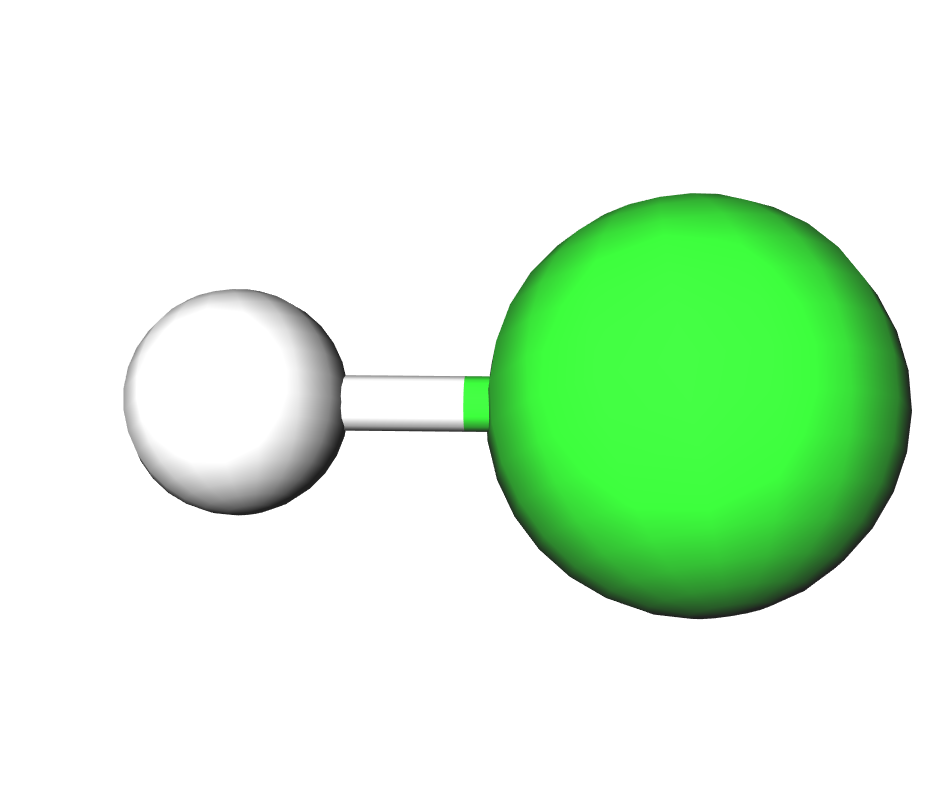 | |||
IUPAC | Hydrochloric acid | |||
INCI | HYDROCHLORIC ACID | |||
CAS | 7647-01-0 | |||
Molar mass | 36,5 g/mol | |||
Density | 10 % - 1,048 kg/l 20 % - 1,098 kg/l 30 % - 1,149 kg/l 32 % - 1,159 kg/l 34 % - 1,169 kg/l 36 % - 1,179 kg/l 38 % - 1,189 kg/l | |||
Solubility | In water: 0 °C - 82.3 g/100 g 30 °C - 67.3 g/100 g 40 °C - 63.3 g/100 g 50 °C - 59.6 g/100 g 60 °C - 56.1 g/100 g | In methanol: -10 °C - 54.6 g/100 g 0 °C - 51.3 g/100 g 20 °C - 47.0 g/100 g 30 °C - 43.0 g/100 g | In ethanol: 0 °C - 45.4 g/100 g 10 °C - 42.7 g/100 g 20 °C - 41.0 g/100 g 30 °C - 38.1 g/100 g | In ether: -10 °C - 37.52 g/100 g 0 °C - 35.6 g/100 g 20 °C - 24.9 g/100 g 30 °C - 19.47 g/100 g |
Hydrochloric acid is a strong acid produced by dissolving HCl gas in water. It is used in the manufacture of plastics such as PVC, pharmaceuticals, the food, leather and metalworking industries, and the extraction of precious metals. It is a constituent of gastric juice. Since the solubility of HCl gas in water is limited, concentrated hydrochloric acid is considered to be a solution of about 33 %. Special conditions are required to achieve and maintain concentrations above 40 %.
In metalworking, hydrochloric acid (salt acid) is a strong inorganic acid used in many industrial processes such as metal refining. One of the uses of hydrochloric acid is the quenching of steel to remove rust or iron oxide precipitates from the iron or steel prior to subsequent processing of the metal (extrusion, rolling, galvanizing etc.). A 14 % concentration of acid is normally used. It is used in hydrometallurgy and electroforming, for cleaning the surface of metals during soldering and preservation, and for the production of chlorides of zinc, manganese, iron and other metals. In a mixture with surfactants, it is used to clean and disinfect ceramics and metal products (where a suppressed acid is needed) from contamination.
In water filters hydrochloric acid can also be used to regenerate ion exchangers. High quality hydrochloric acid is used to regenerate ion exchange resin. Cation exchangers are widely used to remove ions such as Na+ or Ca2+ from aqueous solutions in the preparation of demineralized water. Acid is used to leach the cations from the resin. Sodium ions are replaced by one hydrogen ion and calcium by two. Ion exchangers and demineralized water are used in all chemical industries, in the production of drinking water and in many food industries.
In the production of organic compounds, hydrochloric acid is used as one of the ingredients in the production of plastics, such as vinyl chloride and dichloroethane, which are needed for the production of PVC. It is also used to produce bisphenol A (BPA), which is needed for the production of polycarbonates, activated carbon (wood + HCL + heat = activated carbon), ascorbic acid, as well as for the production of many other pharmaceutical products. Hydrochloric acid of technical purity is unsuitable for the production of organic compounds because of its low concentration and the impurities present.
In water improvement and waste water treatment, hydrochloric acid can be used as a pH-controlling agent (e.g. pool care product pH minus) or as a pH-neutralizing agent. In industries that require a high level of purity (food, pharmaceutical, drinking water industries), high quality hydrochloric acid is used to control the pH of process water streams. In less demanding industries, technical grade hydrochloric acid is sufficient to neutralize waste and control the pH of the pool. Hydrochloric acid is also used to restore the water content of water boreholes, as its injection into the borehole dissolves clogged and calcified water channels and increases the flow of water into the borehole.
In the household, hydrochloric acid can be used as a cleaning agent for kitchen and bathroom tiles and for cleaning toilets. It is also suitable for cleaning porcelain. In winter, when mixed with lime, the resulting salt can be applied in the yard where ice forms to melt it.
In construction hydrochloric acid has been used to dissolve calcium carbonate, i.e. to clean mortar, rust and interior decoration, but it is a hazardous liquid that must be used with care. When cleaning brickwork, the reaction with the mortar only continues until all the acid has been converted to calcium chloride, carbon dioxide and water, so if the desired result is achieved, it is recommended that the surface is rinsed with an alkaline (baking soda) solution to prevent damage to the surfaces being cleaned.
In schools, hydrochloric acid is used to produce hydrogen gas by reacting with zinc. There are several variations: used in a Kip's apparatus, where a constant supply of hydrogen is required, or in test tubes to produce a shot/crackle/whistle sound.
Other uses of the acid: hydrochloric acid is used in many small-scale operations such as skin treatment, purification of table salt. Many of the chemical reactions involving hydrochloric acid are used in the production of food, food ingredients and food additives. Typical products include aspartame, fructose, citric acid, lysine, hydrolyzed vegetable proteins and the production of gelatine, flour processing, etc.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H290 May corrode metals. H314 Severely burns skin and damages eyes. H335 May cause respiratory irritation. |
Precautionary statements: P234 Store only in the original container. P260 Do not inhale dust/fumes/gas/vapors/aerosol. P280 Wear protective gloves/protective clothing/eye/face protection. P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Immediately remove/remove all contaminated clothing. Wash skin with water/rinse. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P304+P340 INTRODUCTION: Remove the victim to fresh air; he/she must be calm and in a position to breathe freely. P309+P311 In case of exposure or feeling unwell: Call the ACCIDENT CONTROL AND INFORMATION OFFICE or seek medical attention. P501 Dispose of contents/container in accordance with national legislation. |
Related products
(8 other products in the same category)












