OXALIC (SORREL) ACID 99%, kg
5.40 €
CHEM056
wood bleach, CAS 144-62-7, oxalic acid, INCI oxalic acid
Parameter | Attribute |
Oxalic acid | Wood bleach, crab acid, (Carboxyl)carboxylic acid, carboxylformic acid, dicarboxylic acid, diformic acid |
Formula | C2H2O4 |
Structure | 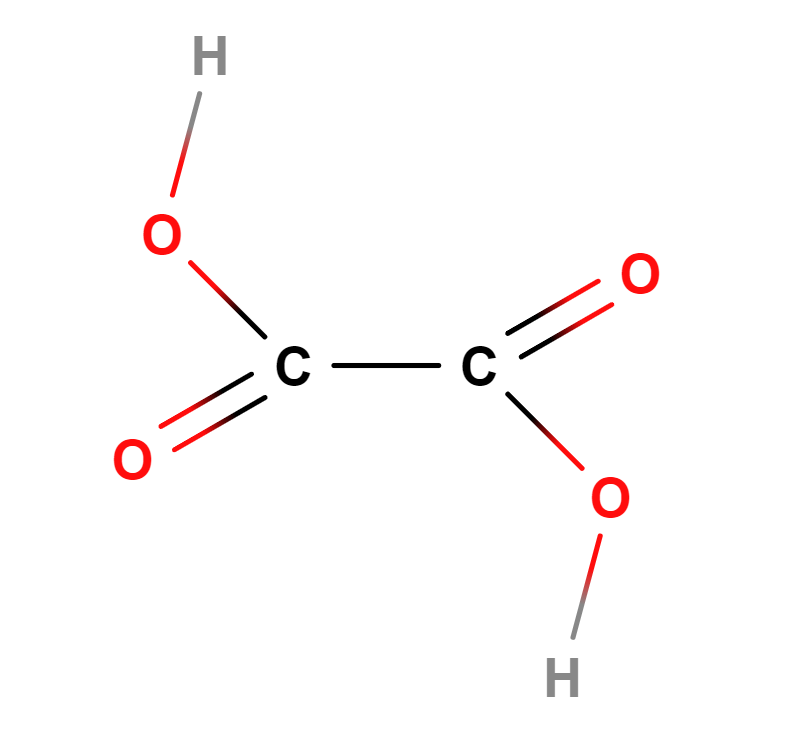  |
IUPAC | Oxalic acid; Ethanedioic acid |
INCI | OXALIC ACID |
CAS | 144-62-7 |
Molar mass | 90,034 g/mol (anhydrous) 126,065 g/mol (dihydrate) |
Density | 1,90 g/cm3 (anhydrous, 17 °C) 1,653 g/cm3 (dihydrate) |
Solubility | In water 90-100 g/l (20 °C); In ethanol 237 g/l (15 °C); In diethylether 14 g/l (15 °C) |
Oxalic acid is also known as ethanoic acid or oxalic acid. It is a relatively strong organic acid, 10 000 times stronger than acetic acid. Oxalic acid and its salts - oxalates - are very common compounds found in many plants. Monocalcium oxalate is found in sorrel, hare's cabbage and rhubarb. Calcium oxalate is particularly abundant in aquatic plants, fungi, ferns and lichens. A little of it is always found in human urine and is often present in bladder and kidney stones.
In water treatment, oxalic acid is used as a descaler for drinking water systems. The popularity of its use is due to its very high efficiency (two acid groups), low damage to metallic piping components compared to mineral acids, non-toxicity and relatively low cost. Oxalic acid can also be used in waste water treatment to bind calcium ions.
In cleaning, oxalic acid is used to bleach white linen and to remove stains, especially fish or grease stains from fabrics. It is also used for cleaning and brightening stone, monuments, old wood and metal surfaces.
In the cosmetic industry, oxalic acid is used in acidic exfoliants, either as one of the main ingredients or as an auxiliary ingredient to stabilise the cosmetic product when used in hard water. Maximum levels in professional products up to 5%. INCI function:
- Chelating: Reacts and forms complexes with metal ions which may affect the stability and/or appearance of cosmetic products.
In metalworking, oxalic acid readily binds iron ions to form oxalates, which are water-soluble and have chelating properties. It can be used both as a cleaner and as a scrubbing agent.
In the wood industry, oxalic acid is used for bleaching wood, in paper bleaching, and for brightening straw.
In the paint industry, it is used to prepare the surface before painting to dissolve/deposite old paint.
In beekeeping, it is used for the treatment of varroa (varicella) both in solution and by evaporation dry. In beekeeping, a 3,5 % acid solution is used for the control of Varroa destructor mites. A solution of glycerol and oxalic acid is usually made so that the bees will go to it willingly (glycerol has a sweet taste), and at the same time be enveloped in oxalic acid. Glycerol is also a moisture-absorbing substance that keeps oxalic acid on the bees for a much longer period of time, thus increasing the effectiveness of the treatment.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: DANGER |
Hazard icons:
|
Danger phrases: H302 Harmful if swallowed. H312 Harmful in contact with skin. |
Precautionary statements: P264 Wash hands thoroughly after use. P280 Wear protective gloves/protective clothing/eye/face protection. P301+P312 CAUTION: If you feel unwell, call the Poison Control and Information Bureau or seek medical attention. P302+P352 IN CASE OF CONTACT WITH SKIN: Wash with plenty of soap and water. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P501 Dispose of contents/container in accordance with local regulations. |
CHEM056
Related products
(8 other products in the same category)












