CALCIUM HYDROXIDE (slaked lime), 90%, kg
1.85 €
Slaked lime, CAS 1305-62-0, milk of lime, INCI CALCIUM HYDROXIDE, calcium(II) hydroxide, pickling lime, hydrated lime, portlandite, calcium hydrate.
Parameter | Attribute |
Calcium hydroxide | Slaked lime, milk of lime, calcium(II) hydroxide, pickling lime, hydrated lime, portlandite, calcium hydrate, calcium dihydroxide |
Formula | Ca(OH)2 |
Structure | 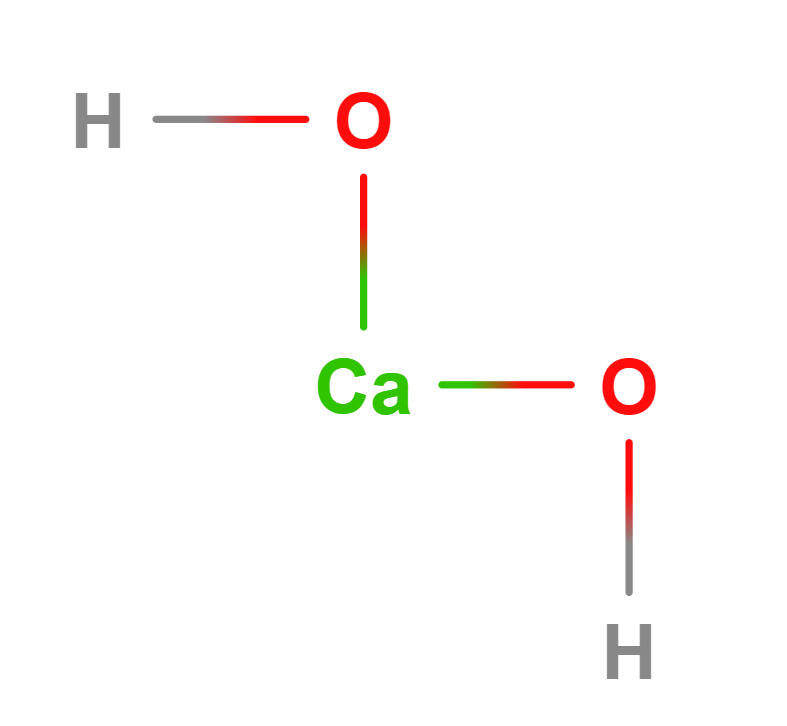 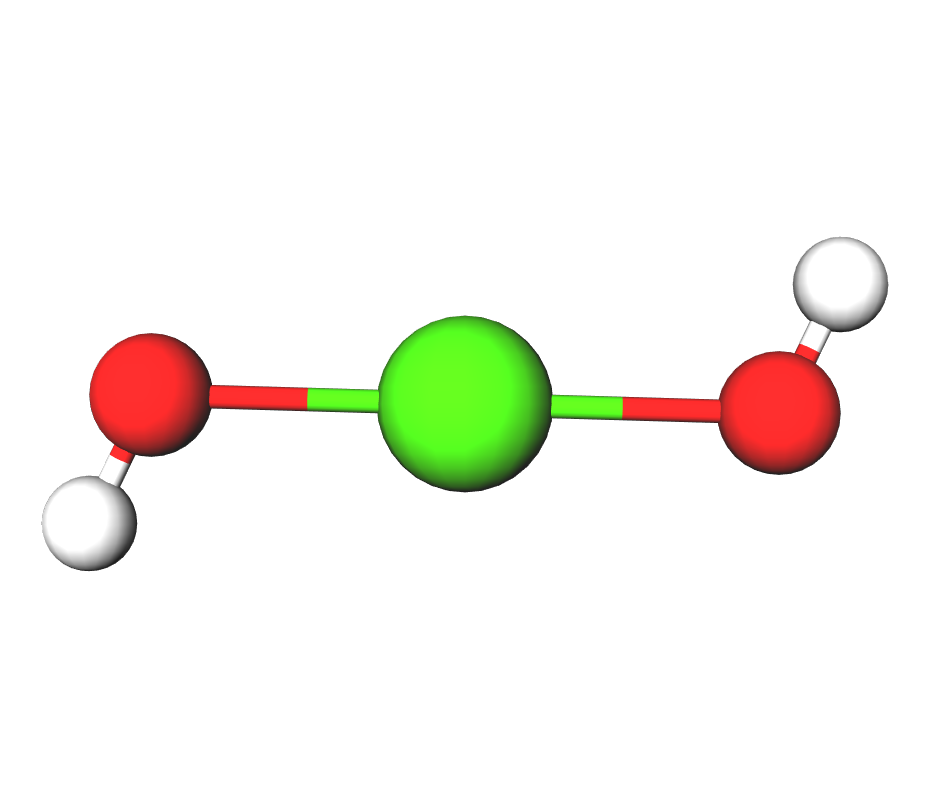 |
IUPAC | Calcium dihydroxide |
INCI | CALCIUM HYDROXIDE |
CAS | 1305-62-0 |
Molar mass | 74,093 g/mol |
Density | 2,211 g/cm3 |
Solubility | Soluble in glycerol and acids (reacts with acidic media), insoluble in alcohols. In water: 1,89 g/l (0 °C) 1,73 g/l (20 °C) 0,66 g/l (100 °C) |
Calcium hydroxide (traditionally known as quicklime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when calcium oxide (called lime or quicklime) is mixed with water. It has many names, including hydrated lime, alkaline lime, or pickling lime. Calcium hydroxide is used in many applications, including food preparation where it has been identified as E number E526. Lime water is a common name for calcium hydroxide solution.
In water treatment, the use of calcium hydroxide is as a flocculant, in water and wastewater treatment. It forms a branched, charged system that attracts fine particles, thereby removing them from the water, resulting in a clearer product. The low cost and low toxicity of calcium hydroxide make it a useful technique. It is also used in freshwater treatment to raise the pH of the water so that pipes do not corrode when the water flow is acidic, as it is self-regulating and does not raise the pH too much.
In livestock farming, slaked lime is used as a feed additive to enrich the diet with the trace element calcium. This method of feeding results in a lower acidity of the feed and in the addition of the trace element calcium. It should be noted that calcium uptake is not very high and it is therefore advisable to use chelating compounds in addition to the calcium to improve calcium uptake.
In horticulture, slaked lime is used to acidify the soil. It is recommended to lime in autumn, as not all vegetables like freshly limed soil. If this is done before the spring sowing, the lime mixes with the soil, becomes more effective and has time to neutralize the acidic soil. The result can be even better if liming the precursors. Cauliflowers, cabbages, beetroots and cauliflowers tolerate immediately limed soils quite well. Carrots, cucumbers, tomatoes and legumes do not like lime. Their seeds should only be added to limed soils once the substances have been neutralized. Potatoes in particular do not like lime and become scabby. Calcium hydroxide is used to bleach garden trees, i.e. to protect them from pests.
In the food industry, calcium hydroxide is used quite extensively, both in processing and in finished products. In the sugar industry, to purify the sugar solution and to obtain white sugar crystals. In the beverage industry to produce clear juices and other beverages made from natural raw materials. In pickled/preserved products to keep them transparent. In the preparation of Chinese aged eggs. In the processing of maize to facilitate the removal/softening of the seed coat. Calcium supplement in beverages, infant formula. In carbon dioxide absorption. As an analogue of baking powder or as an ingredient. For the mixture of calcium and magnesium salts in both the food and pharmaceutical industries.
In the construction industry, calcium hydroxide is used in the formulation of various construction mixtures where it gives a white color effect, smoothness and a faster setting/drying effect. Hydrated lime is used for dry mixes. Lime can be used to stabilize the soil during road construction, where the moisture content of the soil is reduced by using slaked lime (about 3 %). This technique is very common abroad and is quite effective. The amount of lime to be added depends on the moisture content of the soil. The use of lime not only reduces the moisture content of the soil, but also improves the structure and increases the strength. If the soil is weak, lime needs to be applied and mixed to a depth of 40 cm. For example, before starting work on shopping centers or terminals, the soil is levelled, the base is prepared and the whole area is stabilized in its entirety.
In the cosmetics industry, calcium hydroxide is also known as 'slaked lime' or 'hydrated lime'. This element is formed with water and lime. Lime water is the main ingredient in the manufacture of oleo-lime liniments. Calcium hydroxide is found in hair straighteners and has the ability to change the structure of the hair: it breaks the sulfide bonds that bind the amino acids in the hair shafts and changes their physical structure. Finally, it is also used in some products as a PH regulator because it is essentially alkaline.
In laboratories, calcium hydroxide is used to produce ammonia gas (NH3) using the following reaction: Ca(OH)2 + 2NH4Cl → 2NH3 + CaCl2 + 2H2O
In the leather industry, calcium hydroxide is used in leather cleaning to separate fur or residues from the skin.
In the paper industry it is an intermediate in the reaction to produce sodium hydroxide. This conversion is part of the Kraft pulping process for the production of pulp. In the caustication operation, the burnt lime is added to a green liquor, which is essentially a solution of sodium carbonate and sodium sulphate, produced by dissolving the emulsion, which is the molten form of these chemicals, from the utilization furnace.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H315 Irritating to skin H318 Severely damages eyes H335 May cause respiratory irritation |
Precautionary statements: P260 Do not inhale dust. P280 Wear protective gloves/eye protection. P302+P352 In case of contact with skin: wash with plenty of water. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P310 Call the ACCIDENT CONTROL AND INFORMATION OFFICE/ consult a doctor immediately. |
Related products
(8 other products in the same category)












