|
Parameter |
Attribute |
|
Ammonium sulfate |
Diammonium sulfate, diammonium salt of sulfuric acid, mascagnite, dolamine |
|
Formula |
(NH4)2SO4 |
|
Structure |
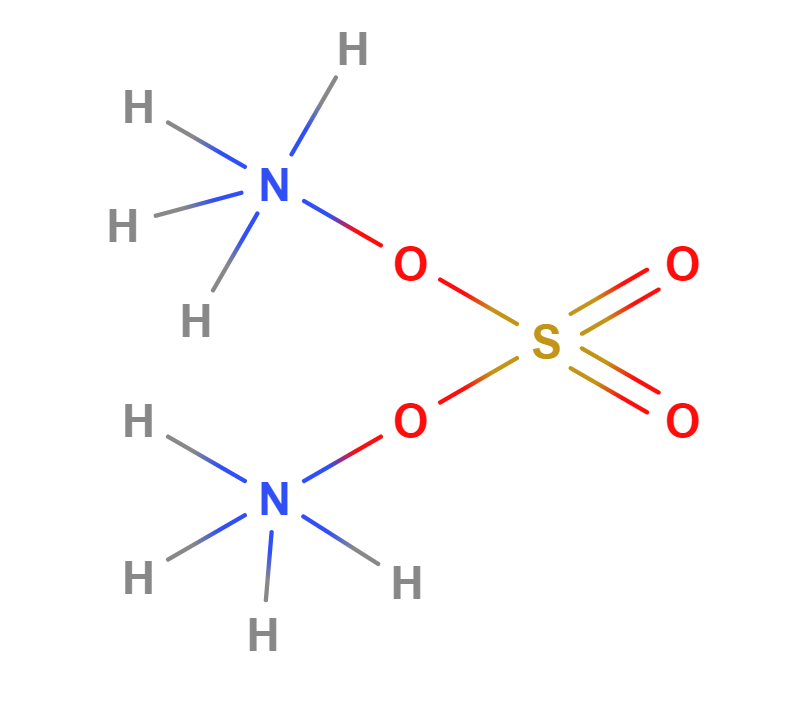  |
|
IUPAC |
Ammonium tetraoxosulfate |
|
INCI |
AMMONIUM SULFATE |
|
CAS |
7783-20-2 |
|
Molar mass |
132.14 g/mol |
|
Density |
1.77g/cm3 |
|
Solubility |
In water: 70.6 g /100 g (0 °C) 74.4 g / 100 g (20 °C) 103.8 g / 100 g (100 °C) |
Ammonium sulfate is an inorganic salt with a wide range of applications. It is most commonly found as a fertilizer with 21% nitrogen and 24% sulfur.
In horticulture and on farms, ammonium sulfate is used as a fertilizer for alkaline soils. The ammonium ion is released in the soil and a small amount of acid is formed, which lowers the pH of the soil, while at the same time the ammonium ions are used by the plant as a source of nitrogen. The biggest negative effect is the relatively low nitrogen content compared to ammonium nitrate or urea, which increases the cost of the fertilizer during transport. Ammonium sulfate is also used as an additive when spraying water-soluble insecticides, herbicides or fungicides. The main function of diammonium sulfate is to chelate (bind calcium and iron ions) in order to soften the applied water and to facilitate the absorption and efficacy of the applied products in the plant cells. Ammonium sulfate partially increases the effectiveness of herbicides such as 2,4-D (amine), glyphosate, glufosinate Ammonium sulfate is ammoniacal in nature, so nitrogen is taken up more slowly by plants and is difficult to leach into the deeper layers of soil. For plants that do not like acidic soils, do not use the product in the same place for a long period of time, as this lowers the pH of the soil. Ammonium sulfate is used in soils with normal and acidic reactions and acidifies them, so it is suitable for plants that like acidic soils, such as hydrangeas, rhododendrons, ericas, heathers, gaultherias, azaleas or hawthorn trees. Apply 30 g of ammonium sulfate evenly over an area of 1 m2 by dry application and water well. For liquid spraying, dissolve 50 g of ammonium sulfate in 10 l of water and apply evenly over an area of 2-3 m2 .
In the food industry, ammonium sulfate is used as an acidity regulator in flour and bread products. More commonly known as food additive E517.
In water improvement, ammonium sulfate is used to disinfect drinking water in combination with chlorine to produce monochloramine, which is less aggressive than free chlorine and considerably more stable under light than hypochlorites.
In fire extinguishing agents ammonium sulfate is used as a flame retardant. Its action is very similar to that of diammonium phosphate. It increases the flash point, reduces weight loss and increases the ember (char) content. Efficiency can be increased by mixing ammonium sulfate with ammonium sulfamate. It is also used in aerial fire extinguishing agents.
In laboratories, ammonium sulfate is used in the production of other ammonium salts, most commonly ammonium persulfate. Ammonium sulfate can also be used to grow brownish crystals.
In the wood industry, ammonium sulfate is used as a wood preservative, but due to its hygroscopic, metal-corrosive properties, instability of parameters, and woodworking problems, its use has been reduced very significantly. It is also used in woodworking as a means of improving the adhesion of the wood surface to the glue. Ammonium sulfate can be found both as a stand-alone component for the preparation of the wood surface prior to gluing and as an ingredient in the wood glue itself.
In the cosmetic industry, ammonium sulfate is used as a thickener in water-based products. When a product is formulated in the desired formulation, e.g. shampoo, shower gel, etc., ammonium sulfate is added at the end of the formulation to adjust the consistency and, in some cases, the pH. Its functions (INCI)
- Viscosity control: increases or decreases the viscosity of cosmetics
In the cleaning industry, ammonium sulfate is used as a bleaching agent in detergents. During washing, dissolved ammonium ions are converted into ammonia, which in turn whitens and brightens fabrics and helps to remove dirt.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
|
Signal word: Not applicable |
|
Hazard icons: Not applicable |
|
Danger phrases: Not applicable |
|
Precautionary statements: Not applicable |
Related products
(8 other products in the same category)










