SODIUM HYDROSULFITE (Sodium dithionite), 88%, kg
5.89 €
Sodium dithionite, CAS 7775-14-6, SDT, D-Ox, Hydrolin, Reductone, sodium hydrosulfite, sodium sulfoxylate, Sulfoxylate Vatrolite, Virtex L Hydrosulfite.
Parameter | Attribute |
Disodium dithionite | Sodium dithionite, SDT, D-Ox, Hydrolin, Reductone, sodium hydrosulfite, sodium sulfoxylate, Sulfoxylate Vatrolite, Virtex L Hydrosulfit, Prayon Blankit, Albite A, Konite Zepar, Burmol, Arostit |
Formula | Na2S2O4 |
Structure |  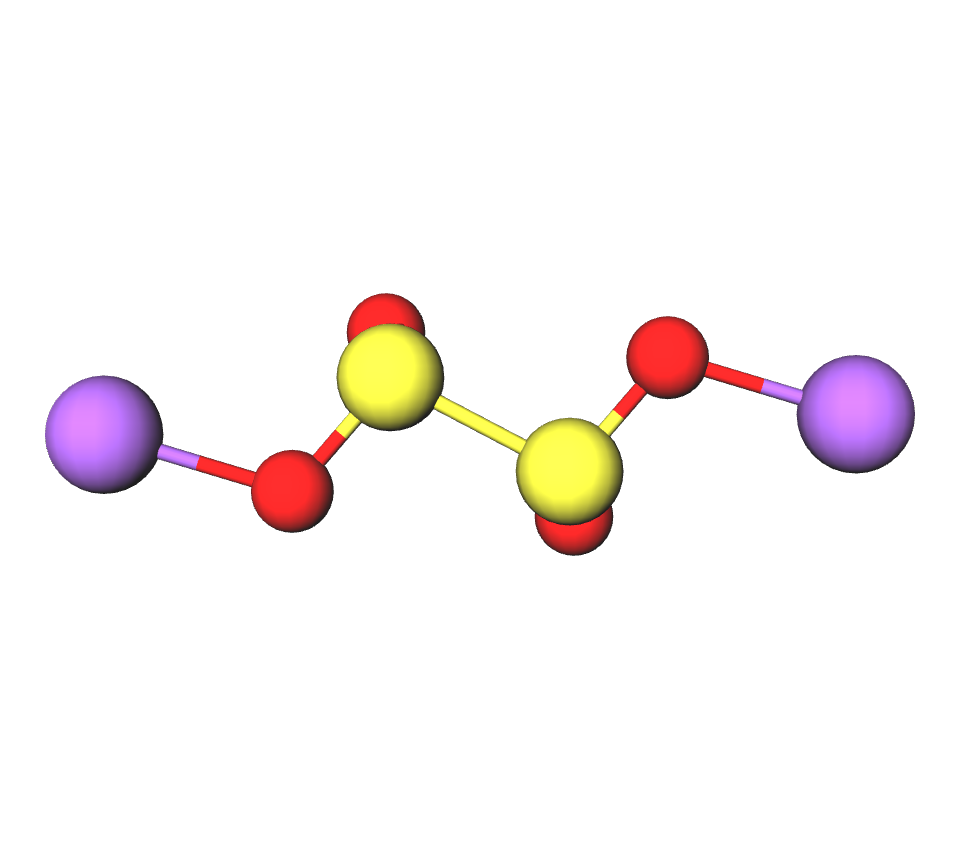 |
IUPAC | Disodium dithionite |
CAS | 7775-14-6 |
Molar mass | 174.107g/mol |
Density | 2,38 g/cm3 (anhydrous) |
Solubility | In water 30,0 g/100 ml (20 °C) |
Sodium dithionite (also known as sodium hydrosulphite or SDT) is a white crystalline powder with a faint sulfur odor. Although it is stable in the absence of air, it decomposes in hot water and acid solutions.
In the cleaning industry, sodium dithionite is used in the home as a bleach for bleached laundry, as a dye remover when laundry has been inadvertently stained with a colored item, and in a high temperature wash cycle. Typical use is about 5 grams per wash. It is found as an ingredient in detergents or bleaching agents.
In water improvement, sodium hydrosulfite is used as a de-oxygenating agent to purify the water supplied to steam boilers to prevent corrosion problems. It is also found in water treatment products for clarification and softening filters. As sodium dithionite is a strong reducing agent, it reduces oxidized iron (rust and brown scale) to an ionic state (making it soluble in water) during the washing cycle, thus removing both filter charges and the cationic and anionic resins present in the softening filters. Often found in "IRONOUT" type cleaners in both powder and liquid form.
In cosmetics, sodium hydrosulfite is used as an antioxidant and as a reducing agent to keep products stable, color and odorless. The reducing properties also allow the control of the thickness of products.
In the photographic industry, SDT is used to protect developer solutions from oxidation and to leach the fixative (sodium thiosulphate) from film and photopaper emulsions.
In textiles it is used as a bleaching, desulfurising and dechlorinating agent. In the leather trade it is used to sulphonate tanning extracts. In the leather industry, it is used as a highly effective bleaching agent and pigment disintegrator. In textile and fabric preparation, SDT is used as a solvent remover for the reduction of insoluble pigments to soluble ones, and for the bleaching and lightening of very strong pigments such as indigo.
In metalworking, SDT is used to remove silver, copper and iron oxides from antique and historical finds. Due to its reducing properties, sodium dithionite converts insoluble metal oxides into soluble ones, and at the same time, due to the alkaline medium and the binding of oxygen, it acts as a corrosion inhibitor which prevents the metals from becoming oxides again.
In fisheries, sodium dithionite is used to regulate fish activity.
In the chemical industry, sodium hydrosulfite is also used to purify laboratory reagents. It is used in chemical production as a sulfonation and sulfomethylation agent.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H251 Self-igniting, potentially flammable. H302 Harmful if swallowed H319 Causes severe eye irritation. |
Precautionary statements: P235+P410 Store in a cool place. Keep out of sunlight P280 Wear protective gloves/protective clothing/use eye/face protection P301+P312 CAUTION: If you feel unwell, call the Poison Control and Information Bureau or seek medical attention. P302+P352 IN CASE OF CONTACT WITH SKIN: Wash with plenty of soap and water. P305+P351+P338 IN EYES: Wash gently with water for a few minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P420 Keep away from other materials EUH031 Produces toxic gases on contact with acids. |
Related products
(8 other products in the same category)












