SULFURIC ACID (tech grade), 98%, L
5.00 €
Sulfuric acid, CAS 7664-93-9, hydrogen sulfate, INCI SULFURIC ACID, Oil of vitriol, Acidum sulfuricum, Acide sulfurique.
Parameter | Attribute |
Sulfuric acid | Sulphuric acid, sulfatic acid, hydrogen sulphate, oil of vitriol, acidum sulphuricum, acide sulphurique |
Formula | H2SO4 |
Structure | 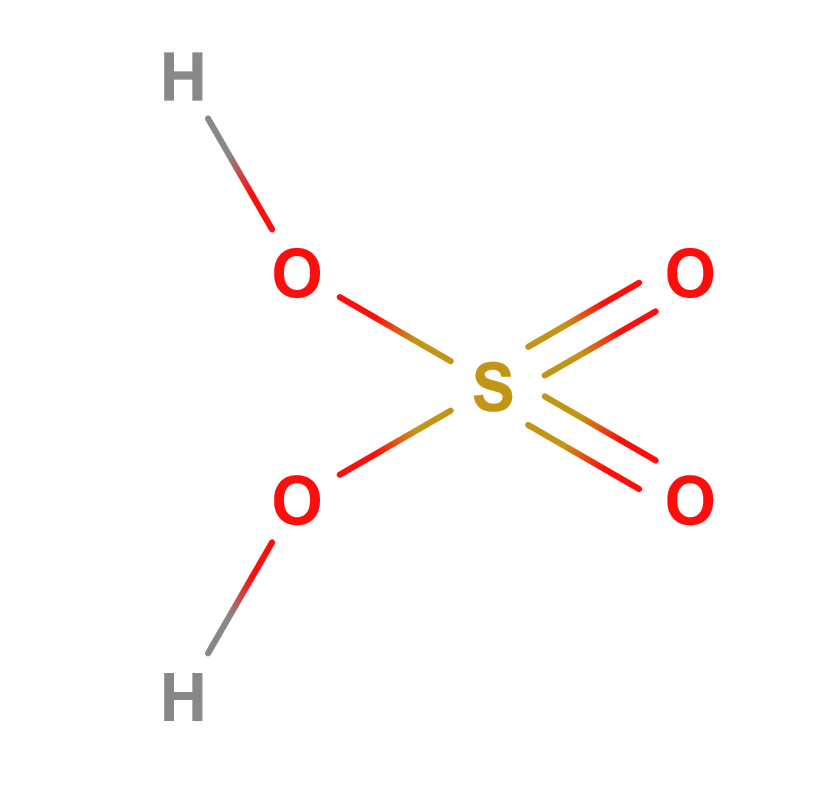  |
IUPAC | Sulfuric acid (Tetraoxosulphate (VI) acid) |
INCI | SULFURIC ACID |
CAS | 7664-93-9 |
Molar mass | 98,079 g/mol |
Density | 1.8302 g/cm3 (98%) |
Solubility | Miscible with water in any ratio, generates a large amount of heat |
Sulfuric acid is a strong inorganic (mineral) acid with the formula H2SO4. It is a colorless, sometimes slightly yellowish, viscous liquid that mixes with water in all proportions. Sometimes the color may be dark due to the presence of organic impurities or specifically to warn people of the hazardous nature of this liquid.
In agriculture and horticulture, sulfuric acid is used to harvest potatoes. A couple of days before the potato harvest, the potato fields are sprayed with a sulfuric acid solution to blacken the stems and leaves. This dries them out to prevent them being caught by implements during harvesting. For harvesting food crops, an aqueous solution containing a combination of urea and sulfuric acid is used. The molar ratio of the latter is approximately 1:4 to 7:4. This composition of these elements is fast-acting, non-toxic and relatively non-hazardous to equipment and the environment. This method avoids toxic substances in the foodstuff, results in significantly less damage to the crop, and is much faster and more efficient. This combination is particularly suitable for early harvesting of potatoes and beetroot. Very low concentrations of urea and sulfuric acid can cause significant drying of the plant leaves. Higher concentrations tend to promote more rapid desiccation and reduce the risk of loss of active substances from the leaves. Spray solutions with a sulfuric acid content of about 5 % by weight are the most suitable, while solutions with a urea-sulfuric acid combination of 10 % by weight are generally used at lower spray rates. The solutions are usually applied to the leaves at a minimum rate of about 22,5 kg/ha, taking into account the total urea and sulfuric acid content. Faster drainage is obtained with an application rate of about 56 kg/ha, while a rate of about 336 kg/ha tends to kill the leaves of all crop species particularly rapidly. As a general rule, to significantly facilitate crop harvesting, spraying 24 hours or earlier, with dosages ranging from 56 to about 560 kg/ha is the predominant method. In agriculture, sulfuric acid is used to acidify the water used to irrigate plants that like acidic soils.
Acid | Concentration | g/m3 of water neutralized with 50 ppm alkalinity | ml/m3 of water neutralized with 50 ppm alkalinity | Received element |
Sulfuric acid | 98 | 37.98 | 69.88 | 16.1 ppm S |
The use of sulfuric acid to lower the pH of irrigation water is very favorable at the end of a period of intense plant growth, when a low pH is needed but plant growth no longer needs to be stimulated, and the economic aspect is also relevant. In horticulture, sulfuric acid is used to reduce diseases and parasites attacking seedlings by lowering the pH of the soil, thus reducing the growth of micro-organisms in the soil.
In the timber industry, sulfuric acid is used to blacken wood to about 5-10%. The resulting chemical burns are similar in appearance to fire burns, except that in this case it is possible to obtain the desired darkness from light brown to black, and to highlight and maintain the wood grain. Sulfuric acid blackening is much less damaging to the surface than fire burning. It is recommended that a test is carried out in a low visibility area before the wood is treated.
In the metal industry, sulfuric acid is used as a strong acid in the precious metal extraction industry to change the state of precious metals from solid to ionic (liquid), both by dissolution and as a medium for a galvanic cell. In the metal arts, sulfuric acid is used to blacken metals (with iron it forms iron II oxide, which is passive and black in color. This not only produces a black metal color but also provides a protective metal coating. Sometimes sulfuric acid is used in finishing blacksmithing products, such as swords, to bring out the structure of the metal. In metal sculpture, sulfuric acid is used to oxidize various metals to give an aged metal effect. Sulfuric acid solutions are used to passivate the surface of aluminium, i.e. in the production of anodized aluminium.
In laboratories, sulfuric acid is used as a catalyst in various reactions. For example, it is a common acid catalyst for the conversion of cyclohexanone oxime to caprolactam, which is used in the production of nylon. It is used to produce hydrochloric acid from salt using the Mannheim process. In petroleum refining, for example as a catalyst in the reaction between isobutane and isobutylene to produce isooctane, a compound that increases the octane rating of petrol (gasoline).
In the fertilizer industry, sulfuric acid is used in the production of phosphoric acid, phosphates and phosphate fertilizers. Apatites (phosphate minerals) are treated with sulfuric acid to produce calcium sulfate and phosphoric acid and, as intermediate products, calcium hydrogen phosphate and/or calcium dihydrogen phosphate (also known as superphosphate). This converts insoluble minerals into soluble minerals that can already be taken up by plants.
Ca3(PO4)2(s) + 2H2SO4(aq) + 4H2O(aq) → Ca(H2PO4)2(s) + 2CaSO4 ∙ 2H2O(s)
Ammonium sulphate, which is obtained by neutralizing ammonia with sulfuric acid, can be used as a nitrogen fertilizer. This process is usually carried out in steel mills, where ammonia is formed at high temperatures and bound by sulfuric acid, which can be precipitated in powder form. The crystals are brown due to iron impurities.
In batteries, sulfuric acid is used as an acid electrolyte in lead acid batteries. The most common solutions are 33.5% solution in passenger car batteries (1.25-1.28 g/ml) and/or 37.52% solution in industrial machinery batteries (1.285 g/ml)
In water treatment, sulfuric acid is used to adjust the pH as a cheap and very effective tool. By lowering the pH of the water medium to 7, it is possible to eliminate the unpleasant taste of sodium/soda and to increase the disinfection efficiency by using chlorine compounds. It also reduces the risk of corrosion of the systems, since at pH above 9 copper, zinc, aluminium, iron, etc. can start to corrode. The use of sulfuric acid helps to separate the emulsions in the water (oil/water) into separate phases, thus increasing the cleaning efficiency. The use of sulfuric acid reduces the hardness of water (1 mole of sulfuric acid reacts with 2 moles of lime). This acid can also be used in the regeneration of water softening filters to remove metal deposits on the filter cartridge.
In the food industry, sulfuric acid is used as a technical auxiliary and as an additive in E513, in cheese production, as well as in sugar refineries and for the purification of drinking water, in the production of modified starch and in the production of special whey proteins needed for muscle development products. Sulfuric acid can be used in two ways: as a technical excipient or as an additive to E513, which is harmless to health. In the first case, the use of sulfuric acid does not need to be specified. In the second case, it has to be indicated in the list of additives. Sulfuric acid intended for the food industry shall be stored in special containers to avoid contamination. E513 sulfuric acid is strictly separated from the acid used for technical purposes only. Sulfuric acid is not specifically added to food as a preservative. However, as a pH adjusting agent, it is considered as a general purpose food additive and is used to directly control the pH during food processing by inhibiting bacterial and microbial growth. Sulfuric acid is used in the production of food acids (i.e. citric and lactic acids) and in pH adjustment of foods during processing. Sulfuric acid is allowed up to a maximum of 0.014% in alcoholic beverages and 0.0003% in cheese. As a food additive used in the production of modified hop extract and as a pH reducer for modified starch. Also used in the extraction process, specifically sulfuric acid, to adjust the water used to extract fucoidans from brown seaweed. Fucoidan is a sulphated polysaccharide used as an ingredient in food supplements.
In the cleaning industry, sulfuric acid is used in large quantities in the steel industry to remove rust and scale from manufactured steel products. The addition of hydrogen peroxide to the sulfuric acid solution produces a 'Piranha solution', which is very strong, but at the same time very toxic. This mixture is most commonly used to etch electronic components or, in laboratories, to clean glassware. Sulfuric acid can be found in high concentrations in acidic pipe cleaners to dissolve dirt, hair or disintegrate paper wipes.
In schools, sulfuric acid is used as a catalyst in many reactions where it is necessary to remove water from the reaction. These can be nitration, esterification, splitting, drying or other types of reactions. Sulfuric acid is used to remove water from sugars to form a solid carbon structure. C11H22O11(s) → 12C(s) + 11H2O(g). This experiment is also known as the "Black Dragon".
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
**- Implementing Regulation (EU) 2019/1148 of the European Parliament and of the Council of 20 June 2019 on trade in and use of explosive precursors, amending Regulation (EC) No 1907/2006 and repealing Regulation (EU) No 98/2013 (OJ L 186, 11.7.2019, p. 1). The product shall only be sold to Legal Persons, farmers or self-employed persons with a signed authorization to purchase and a legal use.
Signal word: DANGER |
Hazard icons:
|
Danger phrases: H314 Severely burns the skin and damages the eyes. |
Precautionary statements: P102 Keep out of the reach of children. P223 Keep away from any possible contact with water as it is highly reactive and may cause a flash fire. P260 Do not inhale vapors/aerosol. P280 Wear protective gloves/protective clothing/use eye/face protection. P301+P330+P331 IN CASE OF DANGER: Rinse mouth. DO NOT induce vomiting. P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Immediately remove/remove all contaminated clothing. Wash skin with water/urine. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P405 Keep under lock and key. |
Related products
(8 other products in the same category)











