HYDROGEN PEROXYDE ~50%, L
36.00 €
Dioxane, CAS 7722-84-1, perhydrol, INCI HYDROGEN PEROXIDE, perhydroxy acid, dihydrodioxide, oxidized water, peroxane.
Parameter | Attribute |
Hydrogen peroxyde | Dioxidane, Oxidanyl, Perhydroxic acid, 0-hydroxyol, Dihydrogen, dioxide, Oxygenated water, Peroxaan |
Formula | H2O2 |
Structure | 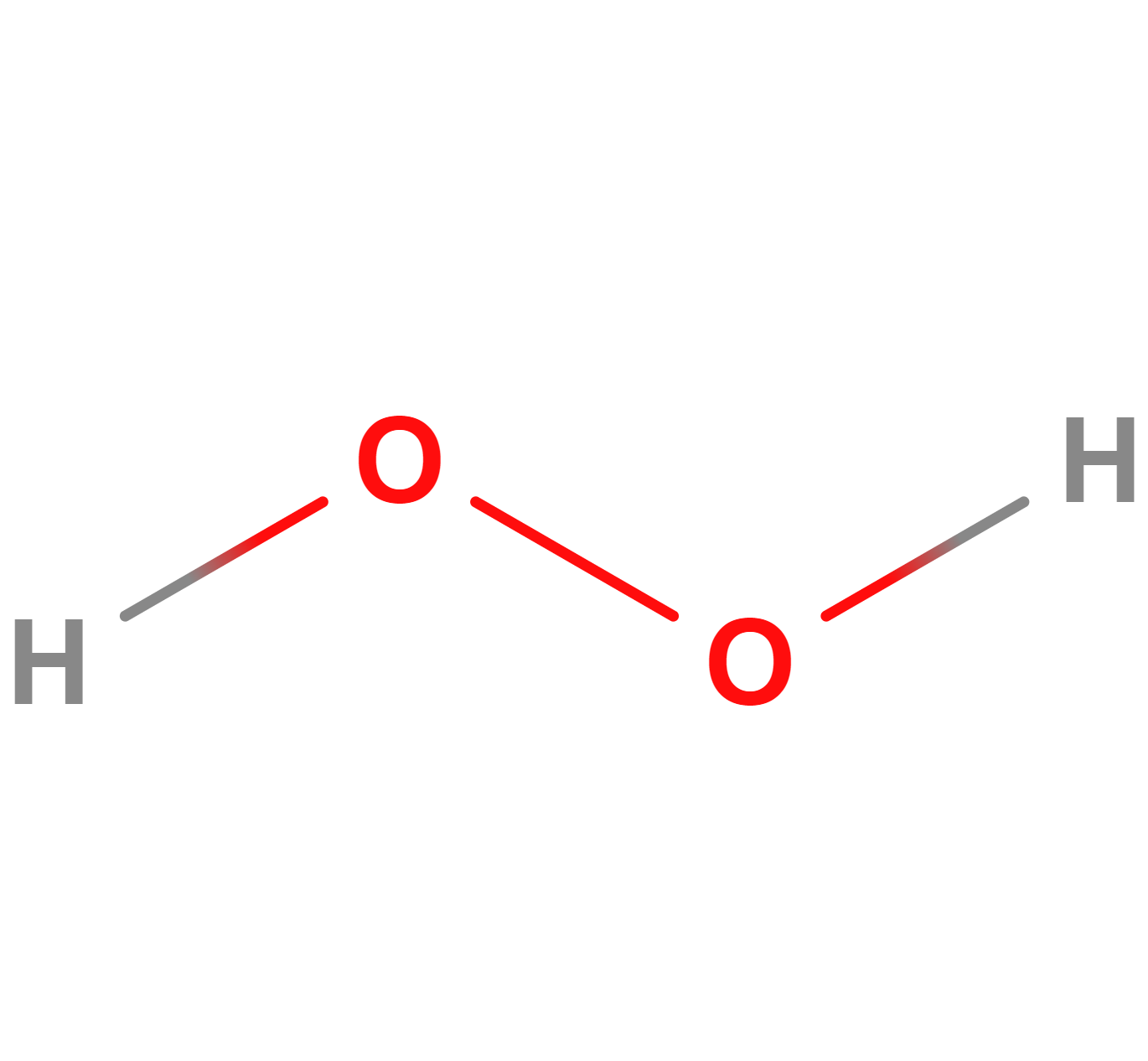  |
IUPAC | Hydrogen peroxide |
INCI | 7722-84-1 |
CAS | HYDROGEN PEROXIDE |
Molar mass | 34,0147 g/mol |
Density | 1,20 g/cm3 (20 °C, 50% (w/w) tirpalas) |
Solubility | Fully miscible with water. Miscible in ethers, alcohols. |
Hydrogen peroxide is a strong oxidant, i.e. a source of oxygen, and therefore has a wide range of applications. It is a colorless, clear liquid, slightly more viscous than water. The compound has the formula H2O2 and a boiling point of 150,2 °C. When hydrogen peroxide is heated without special equipment, it decomposes before it can boil.
In the washing and cleaning industry, hydrogen peroxide is used as a disinfectant for a wide range of surfaces, both as a liquid and as a vapor for disinfecting rooms. Hydrogen peroxide has a broad spectrum activity against viruses, bacteria, fungi, moulds and bacterial spores. It is highly effective against gram-positive bacteria and less effective against gram-negative bacteria because the catalase and peroxidase enzymes present in the bacteria increase their resistance to hydrogen peroxide. Longer contact times and higher concentration solutions (10-30%) are required for sporicidal effect. Hydrogen peroxide is considered a safe disinfectant and is used as an alternative to chlorine-containing products and bleaches because its decomposition produces water and oxygen, which have no long-term adverse effects on the environment. It is recommended that surfaces be mechanically cleaned and rinsed with water before disinfection. The working solution shall be prepared according to the concentration of the solution to be used. In personal health care and public facilities, disinfect water-resistant surfaces with a working solution of 3-5 %, depending on contamination. Wet the surface, spray and leave for 15 min exposure. After disinfection, rinse the surface with potable water. For cleaning glass surfaces, use a 1-5% solution by spraying hydrogen peroxide on the mirror or glass, wait a little and then clean. Cleaning a refrigerator with a 0,5-1% hydrogen peroxide solution easily removes bad odors.
In the food industry, disinfect company tanks, equipment and piping with a 5% working solution. For CIP systems and/or spray systems a concentration of 5% is recommended. Exposure time 30 min. After disinfection, all surfaces should be rinsed with potable water.
In laundries, a chlorine-free fabric bleach is used at a concentration of about 15%, which does not fade the color of the fabrics and maintains a pleasant color and odor. At home, washing and bleaching of fabrics is carried out by rinsing clothes or white linen or towels in water with a glass of hydrogen peroxide. This prevents items from yellowing over time. Hydrogen peroxide also removes stubborn stains. After wetting the stain with hydrogen peroxide, leave it for a minute, brush it thoroughly and rinse it with cold water.
For water treatment and improvement, use for disinfection of hot tubs, swimming pools 1L of 50% hydrogen peroxide solution in 1-2m3 of water in green water or 50-200ml of hydrogen peroxide per week in 1m3 and leave to stand overnight. Hydrogen peroxide kills microbes, algae and unpleasant odors during this time.
In veterinary medicine, hydrogen peroxide is used to disinfect surfaces, milk coolers and milking equipment. A 1-5% solution is used for disinfection of surfaces and a 0.25-2% solution for disinfection of systems with circulating washing.
In medicine a solution of 6-10% concentration used in medicine to disinfect tools, instruments and surfaces. Hydrogen peroxide solution of 1.5-3% concentration for external disinfection. Today, it is believed that disinfection of wounds with hydrogen peroxide slows down healing by breaking down newly formed cells. Only very low concentrations of hydrogen peroxide can be used for wound disinfection. Skin contact with higher concentrations of hydrogen peroxide solution will cause the skin to turn white due to the formation of oxygen bubbles in the capillaries. Alternative medicine uses hydrogen peroxide to treat a wide range of diseases, but in some cases these treatments can be fatal. The use of hydrogen peroxide in medical treatment is debatable: although the body's immune system produces hydrogen peroxide itself, it does so in small quantities. It is produced by phagocytes, which use hydrogen peroxide to kill pathogens. Hydrogen peroxide is toxic to both the pathogen and the cell, and is therefore stored in a special protein in the phagosome. Free hydrogen peroxide affects all the cells it encounters and increases the amount of free oxygen and free radicals in the body, but does not increase the amount of oxygen required for respiration.
In cosmetics, hydrogen peroxide (1,5-12%) is mixed with ammonium hydroxide to produce hair lighteners or hair bleaches, also known as 'peroxide blonde'. Hydrogen peroxide is also used in teeth whitening and is therefore found in many whitening toothpastes. Mixing hydrogen peroxide with sodium chloride and sodium carbonate produces a homemade toothpaste with a whitening effect.
In hunting a 10% solution is used by hunters to whiten the bones of trophy game. Depending on the structure of the bones, the whitening effect may vary.
In the metalworking industry it is used in flux soldering, and in etching of electronic boards. Hydrogen peroxide is also used as an oxidising agent in the extraction of precious metals.
In rockets, hydrogen peroxide as a fuel is used as a potent source of oxygen with a high and rapid expansion, and as a rocket fuel in micro rocket engines. Hydrogen peroxide with a purity of 70-98% is used as the main propellant in rockets. Catalysts used to accelerate the decomposition of hydrogen peroxide include ferric chloride, hydrazine, platinum, silver, molybdenum, palladium, etc. Lower concentrations of hydrogen peroxide are used as a component of rocket fuel. It is usually mixed with kerosene, petrol to produce an instantaneous pulse during rocket launch.
In schools it is used for chemical projects or experiments, it is used to produce vapor or a rich foam ("Elephant's toothpaste"). In glow sticks, it is used as an oxidising agent and energy source. The glow sticks have two compartments. One of them contains hydrogen peroxide. When the separating partition is broken, the reactants mix and react. The hydrogen peroxide reacts with a substance (di-ester, e.g. phenyl oxalate ester) in the other compartment. The reaction produces a substance which immediately decomposes and releases energy, which excites the dye molecules and causes them to emit light.
In horticulture and farming a weak solution of hydrogen peroxide used to water plants is not used in crop production. The peroxide decomposes on the soil when the solution hits the soil, bringing a large amount of oxygen to the roots, improving root development and helping to treat root rot and other diseases.
In aquaculture, it is not used as a source of oxygen for small fish. A weak solution of hydrogen peroxide used by aquarists not only increases the oxygen content of the water, but also helps to disinfect the aquarium from algae without adverse effects on fish. In ponds and fish tanks, it can also be used as an oxygen source and an anti-algae agent
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, colour, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
**- Implementing Regulation (EU) 2019/1148 of the European Parliament and of the Council of 20 June 2019 on trade in and use of explosive precursors, amending Regulation (EC) No 1907/2006 and repealing Regulation (EU) No 98/2013 (OJ L 186, 11.7.2019, p. 1). The product shall only be sold to Legal Persons, farmers or self-employed persons after a signed confirmation of the authorisation to purchase and of the legitimate use by completing a declaration form.
Signal word: DANGER |
Hazard icons:
|
Danger phrases: H272 May increase the risk of fire, oxidiser H302 Harmful if swallowed H332 Harmful by inhalation H314 Severely burns skin and damages eyes H335 May cause respiratory irritation H412 Harmful to aquatic organisms, causes long-term effects |
Precautionary statements: P210 Keep away from heat sources/sparks/open flames/hot surfaces. P261 Avoid inhalation of fumes/vapors. P273 Keep away from the environment. P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Immediately remove/remove all contaminated clothing. Wash skin with water/rinse. P304+P340 INTRODUCTION: Remove the victim to fresh air; he/she must be calm and in a position to breathe freely. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. |
Related products
(8 other products in the same category)
















