CALCIUM SULFATE 99.9%, (gypsum), kg
4.15 €
Calcium sulfate, CAS 10034-76-1, calcium sulfate hemihydrate, INCI CALCIUM SULFATE, Plaster of Paris, drierite, gypsum
Parameter | Attribute |
Calcium sulfate | Plaster of Paris, drierite, gypsum, CI 77231 |
Formula | CaSO4 · 1/2H2O |
Structure | 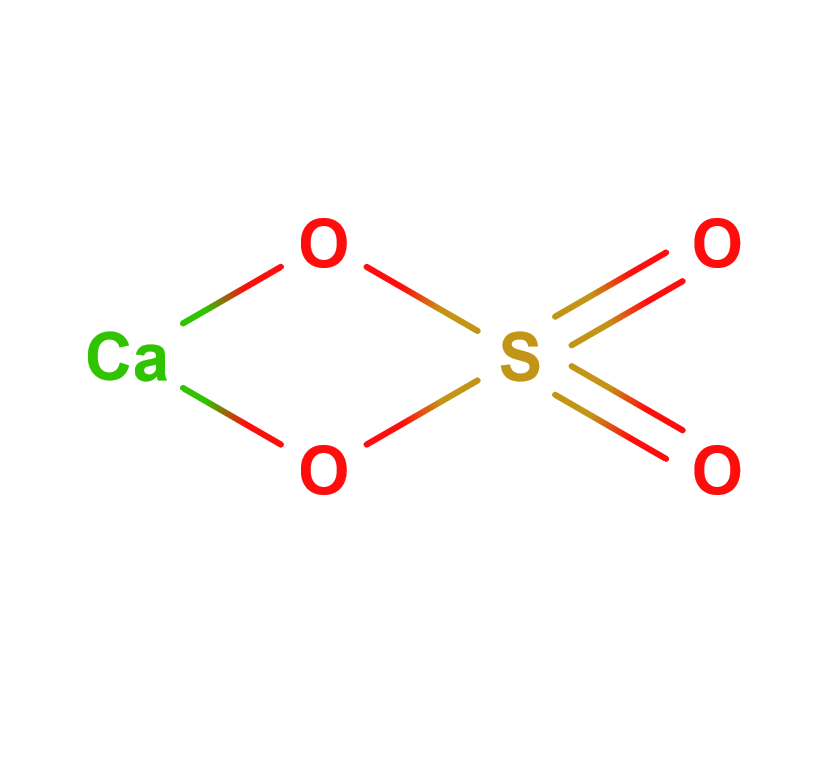  |
IUPAC | Calcium sulfate |
INCI | CALCIUM SULFATE |
CAS | 10034-76-1 |
Molar mass | 74,093 g/mol |
Density | 2,211 g/cm3 |
Solubility | Slightly soluble in glycerin. In water: 0.26 g/100ml 25 °C |
Calcium sulfate is an inorganic compound with the formula CaSO4 and related hydrates. It is used as a desiccant in the form of γ-anhydrite (anhydrous form). The monohydrate is better known as plaster of Paris, and the other naturally occurring form is mineral gypsum. It is widely used in industry. All forms are white solids which are poorly soluble in water. Calcium sulfate causes water hardness.
In ceramics, calcium sulfate is used as a plaster in mould making and modelling, in mould making (for ceramics and other products), as a base for dry plaster and for special gypsum-based aggregate mixes. As a material for sculpture, alabaster was particularly used in the ancient world until the development of steel, when its relative softness made it much easier to carve. In the Middle Ages it was used by scribes and illuminators as an ingredient in gesso, which was used for illuminated letters and gilded in illuminated manuscripts. Gypsum is made from high-quality, light-colored synthetic calcium sulfate and has a high final stability compared to typical hemihydrates. Can be used as a base for gypsum putty. The most important properties of gypsum are: very light, very low content of non-sulfate additives, high quality milling (especially fine), high reactivity, no additives, high final stability. Recommended preparation method: Pour water into a clean container. The volume of water should be slightly less than the volume of the mix already made. Sprinkle the plaster mixture evenly into the water until the mixture is no longer thin and appears on the surface of the water. Stir the mixture until smooth. Once the mixture is ready, fill the desired mould and leave to set for approximately 30 minutes.
In horticulture calcium sulfate is used as a fertilizer. Gypsum provides the two secondary macro-elements of the plant, calcium and sulfur. Unlike limestone, it generally does not affect the pH of the soil and can therefore be used for all types of plants. For the improvement of saline soils, regardless of pH, calcium sulfate is added to sodium (salt) and acidic soils, converting the highly soluble form of boron (sodium metaborate) into the less soluble calcium metaborate. Gypsum reduces the toxicity of aluminum and boron in acid soils. It also improves soil structure, water absorption and aeration.
In the construction industry, calcium sulfate is used in a variety of mortar formulations where it gives a white color effect, smoothness and a faster setting/drying effect. Gypsum gives a degree of fire resistance to plasterboard products and glass fibers are added to enhance this effect. Gypsum has a low thermal conductivity, which gives the plaster some insulating properties. In building construction, plaster blocks are used as concrete blocks. Gypsum mortar is an ancient mortar used in the construction of buildings. A component of Portland cement used to prevent concrete from hardening (setting too quickly).
In the food industry, calcium sulfate is found as a coagulant in Tofu (soybean curd). In brewing, it is used as a water hardness regulator and as a source of calcium for yeast cultures. In the pastry industry, calcium sulfate is used in baked goods as a dough conditioner, to reduce stickiness, and as a source of dietary calcium for baked goods. It is used in mushroom cultivation as a source of minerals. In some cases used in cereal production under wet conditions to prevent the grain from clumping. Reduces the pH of menthal and increases the fixed hardness of water (calcium ions). 1 g/10 l increases the salt level by 23 ppm calcium and 56 ppm sulfate, and increases the fixed hardness of water by 58 ppm.
In water treatment, calcium sulfate is used as a binder for heavy elements (lead, arsenic, etc.) in water and wastewater treatment. It forms insoluble complex compounds, so that the heavy elements are precipitated with the sediment and become inactive This method is used because of the low cost and low toxicity of calcium sulfate.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: not applicable |
Hazard icons: not applicable |
Danger phrases: not applicable |
Precautionary statements: not applicable |
Related products
(8 other products in the same category)










