SODIUM HYPOCHLORITE 15%, 180g/l, L
3.40 €
antiformin, CAS 7681-52-9, bleach, INCI sodium hypochlorite, chloride of soda, Carrel-Dakin solution, modified Dakin's solution, surgical chlorinated soda sol
Parameter | Attribute |
Sodium hypochlorite | Antiformin, bleach, chloride of soda, Carrel-Dakin solution, modified Dakin's solution, surgical chlorinated soda solution |
Formula | NaOCl |
Structure | 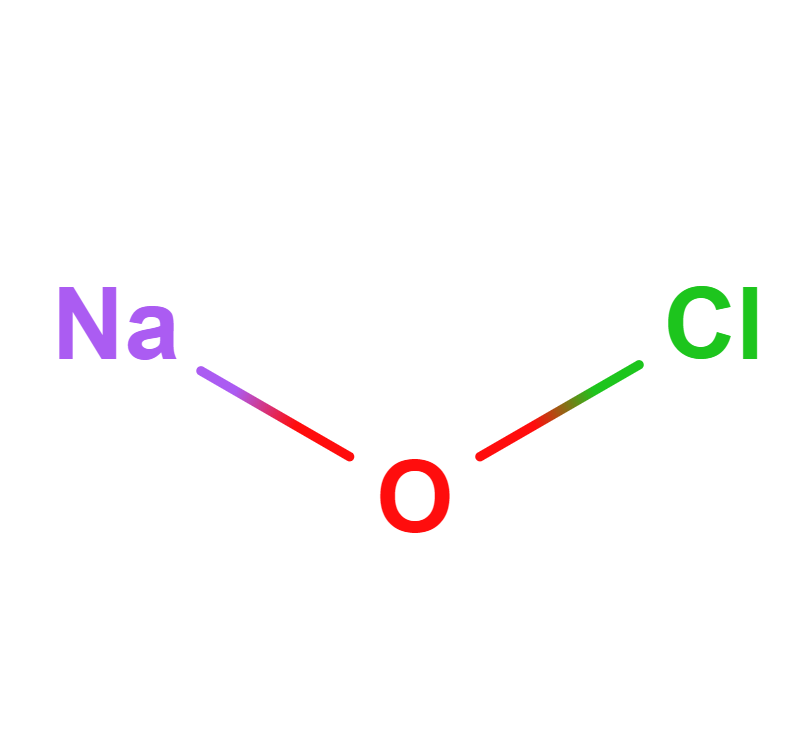  |
IUPAC | Sodium Hypochlorite |
INCI | SODIUM HYPOCHLORITE |
CAS | 7681-52-9 |
Molar mass | 74,442 g/mol |
Density | 1,11 g/cm3 |
Solubility | In water 29,3 g/100mL (0 °C) |
It is the sodium salt of hypochlorous acid with the chemical formula NaOCl or NaClO. It is most commonly found in a liquid state as a greenish-yellow solution and is widely used as a bleaching agent. The anhydrous form of the salt is unstable and may explode. Crystallized to pentahydrate (NaClO-5H2O), a solid greenish-yellow solid which is stable and non-explosive when kept in a cold place.
For bleaching. Home bleach is a solution containing 3-8% sodium hypochlorite and 0,01-0,05% sodium hydroxide. Sodium hydroxide is used to slow the breakdown of sodium hypochlorite into sodium chloride and sodium chlorate.
For cleaning. Among other uses, sodium hypochlorite has good cleaning properties. It can be used to remove mould stains, dental stains caused by fluorosis and to clean stains on crockery, especially those caused by tea tannins. It has also been used in laundry detergents and as a surface cleaning agent.
For disinfection. Sodium hypochlorite in solution has broad-spectrum antimicrobial activity and is widely used in a wide range of health care settings. It is usually diluted with water depending on its intended use. "Strong chlorine solution' is a 0,5 % hypochlorite solution (containing approximately 5 000 ppm free chlorine) used for wound disinfection, where the wound is first cleaned with a detergent before disinfection. Such solutions have been used to deactivate C. difficile and HPV viruses. "A 'weak chlorine solution' is a 0,05% hypochlorite solution used for hand washing. "Dakin's solution is a disinfectant solution containing a low concentration of sodium hypochlorite and a small amount of boric acid or sodium bicarbonate to stabilize pH. The solution has been found to be effective at NaOCl concentrations below 0,025%. Food processing equipment and food contact surfaces may be cleaned with solutions containing bleach, provided that the solution is allowed to drain adequately before coming into contact with the food and that the solutions do not contain chlorine in excess of 200 ppm (e.g., one tablespoon of a typical household bleach solution containing 5.25% sodium hypochlorite per gallon (~3.78 l) of water). If higher concentrations are used, the surface should be rinsed with additional water after cleaning. Similar concentrations of bleach with warm water are used to disinfect beer or wine production tanks. After washing, containers should be rinsed again with boiling water to avoid unwanted odors and chlorinated by-products. Solutions containing more than 500 ppm chlorine are corrosive to some metals, alloys and many thermoplastics (e.g. Acetal resin) and should be carefully disposed of afterwards. For this reason, disinfection with ethanol is sometimes followed by disinfection with bleach. Liquids with sodium hypochlorite as the main active ingredient are also used for household cleaning and disinfection, such as toilet cleaners. Non-dissociated (non-ionized) hypochlorous acid is believed to react with and neutralize bacterial and viral enzymes. Neutrophils in the human immune system produce small amounts of hypochlorite inside phagosomes, which digest (degrade) bacteria and viruses.
To remove unpleasant odors. Along with its cleansing, disinfecting properties, sodium hypochlorite is also effective in eliminating unpleasant odors.
For wastewater treatment. Sodium hypochlorite solutions have been used to treat cyanide-containing wastewaters such as electrolyte surfaces. Sodium hypochlorite is commonly used as a biocide in industry to control slime and bacterial growth in water systems. Used in power stations, pulp and paper mills, etc., in solutions usually at concentrations of 10-15 %.
In endodontics. Sodium hypochlorite is effective against pathogenic organisms during endodontic treatment. Its concentration ranges from 0,5 % to 5,25 %. At low concentrations, it dissolves mainly necrotic tissue; at higher concentrations it also dissolves vital tissue and additional bacterial species. One study showed that Enterococcus faecalis was still visible in the gingiva after 40 minutes of exposure to 1,3 % and 2,5 % sodium hypochlorite, whereas exposure to 5,25 % sodium hypochlorite for 40 minutes was effective in removing E. faecalis. The effectiveness of sodium hypochlorite in removing soft tissue and bacteria in the root canal chamber is not only increased by increasing the concentration of sodium hypochlorite, but also by exposing the area to be removed for a longer period of time or by heating the solution.
Neutralizing nerve agents. Sodium hypochlorite is used to neutralize the effects of nerve agents such as nerve paralyzing gases.
In swimming pool maintenance, sodium hypochlorite shall be used to disinfect the water at a level that provides the required concentration of active residual chlorine in the water.
Name of the object | Residual active chlorine concentration, mg/l |
Water for immersion in indoor swimming, bathing, diving pools, hot water whirlpools, therapeutic, cold water baths | 0,5-2,0 |
Outdoor swimming pools | Up to 3,0 |
Swimming pools for children under 7 years of age | 0,3-1,0 |
The quantity of sodium hypochlorite solution (ml) needed to produce the required concentration of residual active chlorine in 10 m3 of water is calculated as follows:
Active chlorine content, % in sodium hypochlorite solution | Residual active chlorine concentration, mg/l | |||||
0,7 | 1,0 | 1,5 | 2,0 | 2,5 | 3,0 | |
15 | 47 | 67 | 100 | 133 | 167 | 200 |
14 | 50 | 71 | 107 | 143 | 179 | 214 |
13 | 54 | 77 | 115 | 154 | 192 | 231 |
12 | 58 | 83 | 125 | 167 | 208 | 250 |
11 | 64 | 91 | 136 | 182 | 227 | 273 |
10 | 70 | 100 | 150 | 200 | 250 | 300 |
9 | 78 | 111 | 167 | 222 | 278 | 333 |
For disinfection of drinking water production and storage equipment, tanks (reservoirs), means of use and pipelines, working solutions of 50, 70, 150, 250 mg/l are used. Production of working solutions:
Active chlorine content, % in sodium hypochlorite solution | Amount of sodium hypochlorite (ml) needed to produce 1 m3 | ||||
50 mg/l active chlorine in solution | 75 mg/l active chlorine in solution | 100 mg/l active chlorine in solution | 200 mg/l active chlorine in solution | 250 mg/l active chlorine in solution | |
15 | 330 | 500 | 670 | 1330 | 1670 |
14 | 360 | 540 | 710 | 1430 | 1790 |
13 | 390 | 580 | 770 | 1540 | 1820 |
12 | 420 | 630 | 830 | 1670 | 2080 |
11 | 460 | 680 | 910 | 180 | 2270 |
10 | 500 | 750 | 1000 | 2000 | 1500 |
9 | 560 | 830 | 1110 | 2230 | 2780 |
8 | 630 | 940 | 1250 | 2500 | 3130 |
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H290 May corrode metals. H314 Severely burns skin and damages eyes. H335 May cause respiratory irritation. H400 Very toxic to aquatic organisms. EUH031 Produces toxic gases on contact with acids. |
Precautionary statements: P260 Do not inhale dust/ fumes/ gases/ mists/ vapors/ aerosols. P273 Keep out of the environment. P280 Wear protective gloves/ protective clothing/ eye/face protection. P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Immediately remove/remove all contaminated clothing. Wash skin with water/rinse. P305+P351+P338 IN EYES: Wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P310 Call the ACCIDENT CONTROL AND INFORMATION OFFICE or seek medical advice immediately. P403+P233 Store in a well-ventilated place. Keep container tightly closed. |
Related products
(8 other products in the same category)













