SODIUM HYDROXIDE (caustic soda) 50%, L
4.90 €
CHEM028
sodium hydroxide, CAS 1310-73-2, sodium lye, INCI sodium hydroxide, caustic soda
Parameter | Attribute |
Sodium hydroxide | Caustic soda, soda lye, white caustic, sodium hydrate, lye, sodium alkali |
Formula | NaOH |
Structure | 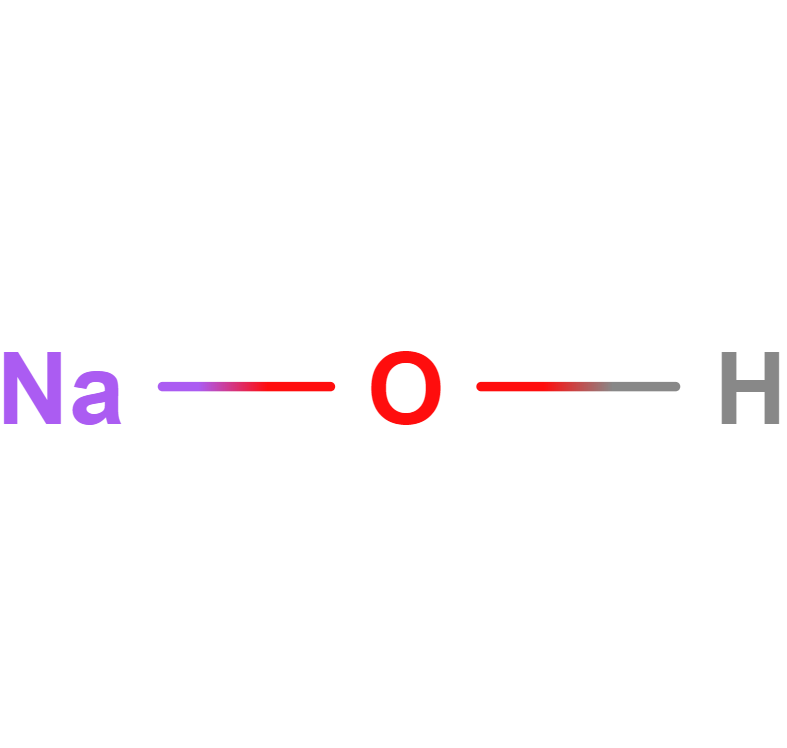  |
IUPAC | Sodium hydroxide |
INCI | SODIUM HYDROXIDE |
CAS | 1310-73-2 |
Molar mass | 39,9971 g/mol |
Density | 2,13 g/cm3 |
Solubility | In water: 418 g/L (0 °C) |
Sodium hydroxide also known as caustic soda or soda lye, it is a strong base with the chemical formula NaOH. Sometimes only the aqueous solution of this compound is referred to as caustic soda. It is used in the manufacture of soap, rayon, paints, petroleum products, and other applications. Sodium alkali can be obtained by electrolysis of an aqueous solution of sodium chloride. In this case, hydrogen is released at the cathode and chlorine at the anode. Sodium hydroxide is a popular strong base used in industry. Approximately 56% of the sodium hydroxide produced is used in industry, of which 25% is used in the paper industry. Sodium hydroxide is also used in the production of sodium salts and detergents, pH adjustment and organic synthesis. It is used in Bayer's aluminum production process. It is most commonly used as an aqueous solution because the solutions are cheaper and easier to handle. Sodium hydroxide is used in many applications where it is desirable to increase the alkalinity of a mixture or neutralize acids. For example, in the petroleum industry, sodium hydroxide is used as a drilling fluid additive to increase the alkalinity of bentonite in fluid systems, to increase the viscosity of the fluid, and to neutralize any acid gases (such as hydrogen sulfide and carbon dioxide) that may be emitted during drilling. Poor quality crude oil can be treated with sodium hydroxide to remove sulfur impurities, a process known as caustic washing. Sodium hydroxide reacts with weak acids such as hydrogen sulfide and mercaptans to form non-volatile sodium salts which can be removed. The waste generated by the process is toxic and difficult to dispose of, and the process is banned in many countries.
Chemical softening of materials. Sodium hydroxide is also widely used to soften wood chips in papermaking. Together with sodium sulfide, sodium hydroxide is the main component of the white liquid solution used to separate lignin from cellulose fibers in the kraft process. It also plays an important role in several subsequent steps in the bleaching process of the pulp obtained after the pulping process. These steps include oxygen delignification, oxidative extraction and simple extraction, all of which require a strongly alkaline environment with a pH > 10,5.
Tissue destruction (recovery). Aqueous sodium hydroxide solution can be used for the disposal of tissues (e.g. dead animals). Tissues placed in sodium hydroxide solution dissolve, the only solid impurities remaining are the bones, which remain so fragile that they can be crushed into flour simply by hand.
In the cosmetics industry, caustic soda is used in the preparation of many products (detergents, salts, precious metals...). It is used to make sodium salts and to neutralize acids. In cosmetics, it allows the production of solid soaps. Caustic soda is allowed in organic production and does not cause problems of classification and regulation. Main functions of INCIs:
- pH adjuster: Stabilizes the pH of cosmetics.
- Denaturant: Makes cosmetics unpleasant. Mainly added to cosmetics containing ethyl alcohol
Dissolving amphoteric metals and compounds. Strong bases affect aluminum. Sodium hydroxide reacts with aluminum and water to produce hydrogen gas. Aluminum takes an oxygen atom from sodium hydroxide, which in turn takes an oxygen atom from water, thus releasing two hydrogen atoms. The reaction generates hydrogen gas and sodium aluminate. In this reaction, the sodium hydroxide acts as a medium to make the solution alkaline in which the aluminum can dissolve. This reaction can be useful in etching processes, in removing anodic coatings or in converting a polished surface into a satin-like finish. "In the Bayer process“, sodium hydroxide is used to refine alumina from ore (bauxite). Alumina is the raw material used to produce aluminum metal by the electrolytic Hall-Héroult process. Since alumina is amphoteric, it dissolves in sodium hydroxide, leaving impurities that are less soluble in alkaline solutions to settle to the bottom of the vessel. Other amphoteric metals are zinc and lead, which are dissolved in concentrated sodium hydroxide solutions to give sodium zincate and sodium plumbate respectively.
Esterification and transesterification reagent. Sodium hydroxide is traditionally used in soap making (cold process soap). It was produced in the nineteenth century in solid rather than liquid form because it was easier to store and transport. In biodiesel production, sodium hydroxide is used as a catalyst for the transesterification of methanol and triglycerides. Only anhydrous sodium hydroxide can be used in these processes, as the combination with water converts the fat into soap, which can be contaminated with methanol. NaOH is used more frequently than potassium hydroxide because it is cheaper and requires less volume.
Food industry. In the food industry, the uses of sodium hydroxide are many and include the chemical washing or peeling of fruit and vegetables, the processing of chocolate and cocoa, the coloring of caramel, the rendering of poultry, the processing of carbonated beverages and the thickening of ice cream. Olives are often soaked in sodium hydroxide to make them softer. Pretzels are glazed with a sodium hydroxide solution before baking to make them brittle.
Cleaning agent. Sodium hydroxide is often used as an industrial cleaning agent. It is mixed with water, heated and then used to clean equipment, containers, etc. It can dissolve grease, oils and fatty-white sludge. It is also used for cleaning domestic waste water outlets (under sinks, toilets, bath/shower). Surfactants may be added to the sodium hydroxide solution to stabilize the dissolved substances and thus prevent secondary contamination when deposits settle on the surface to be cleaned after washing. It is also a common ingredient in oven cleaners. A common use of sodium hydroxide is in the manufacture of detergents for parts. Sodium hydroxide-based parts detergents are some of the most aggressive parts cleaning chemicals. Sodium hydroxide-based detergents include surfactants, rust inhibitors and foaming detergents. Parts washing machines heat water and detergent in a closed housing and then spray the heated sodium hydroxide and hot water under pressure onto the dirt on the parts. Water and sodium hydroxide detergent-based parts washing machines are more environmentally friendly compared to solvent-based cleaning methods. Sodium hydroxide can be used as a drain unblocker in households as it is a good fat solubilizer and also hydrolyses proteins (e.g. hair).
Water purification. Sodium hydroxide is sometimes used in water purification to raise the pH of the supply water. The increase in pH makes the water less corrosive and reduces the amount of lead, copper and other toxic metals that can dissolve in drinking water.
In cement mixtures, mortars, concretes, grouts. Sodium hydroxide is used in some cement mix plasticizers. It helps to homogenize cement mixtures, prevent the separation of sand and cement, reduce the amount of water required in the mixture and increase the workability of the cement.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H290 May corrode metals H314 Severely burns skin and damages eyes |
Precautionary statements: P260 Do not inhale dust/ fumes/ gases/ mists/ vapors/ aerosols. P280 Wear protective gloves/protective clothing/eye/face protection. P301+P330+P331 IN CASE OF DANGER: Rinse mouth. DO NOT induce vomiting P303+P361+P353 IN CASE OF CONTACT WITH SKIN (or hair): Remove all contaminated clothing. Wash skin with water or shower P305+P351+P338 IN EYES: wash gently with water for a few minutes. Remove contact lenses, if present and if easy to do so. Continue to wash eyes. P310 Call the ACCIDENT CONTROL AND INFORMATION OFFICE or seek medical advice immediately. |
CHEM028
Related products
(8 other products in the same category)











