SODIUM CARBONATE (SODA ASH) 99%, KG
2.00 €
Calcined soda, CAS 497-19-8, Soda ash, INCI SODIUM CARBONATE, washing soda, soda crystals.
Parameter | Attribute | |||
Sodium carbonate | Calcinated soda, Soda ash, washing soda, soda crystals | |||
Formula | Na2CO3 | |||
Structure | 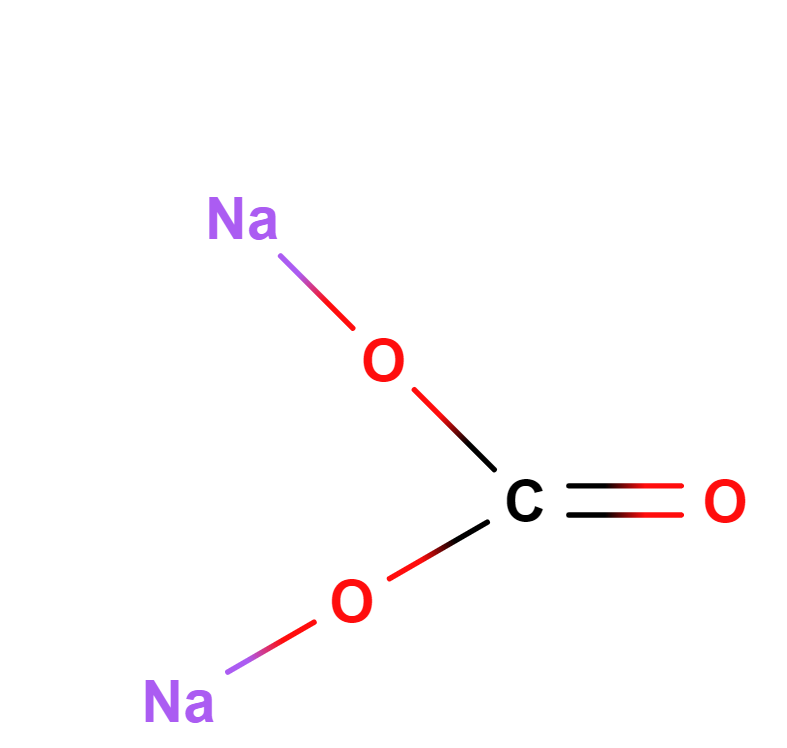  | |||
IUPAC | Sodium carbonate | |||
INCI | SODIUM CARBONATE | |||
CAS | 497-19-8 (anhydrous) | |||
Molar mass | 105,9888 g/mol (anhydrous) 286,1416 g/mol (decahidrate) | |||
Density | Anhydrous: 2.54 g/cm3 (25 °C) 1.92 g/cm3 (856 °C) | |||
Solubility | In water: 7 g/100 ml (0 °C) 16,4 g/100 ml (15 °C) 34,07 g/100 ml (27,8 °C) 48,69 g/100 ml (34,8 °C) 48,1 g/100 ml (41,9 °C) 45,62 g/100 ml (60 °C) 43,6 g/100 ml (100 °C) | In glycerin: 98,3 g/100 g (155 °C) | In ethandiol: 3,46 g/100 g (20 °C) | In dimetilformamide: 0,5 g/kg |
A salt of sodium and carbonic acid with the chemical formula Na2CO3. It is freely soluble in water, giving off heat to form a decahydrate (Na2CO3-10H2O) called washing soda. The name baking soda is derived from the name of a plant salt, Salsola soda, the ash of which was used in the production of sodium carbonate.
In glass making, soda ash is used as a flux to melt silica sand, thereby reducing the melting point. The resulting soda glass is partially soluble, so calcium carbonate is added during the melting process to make the glass insoluble in water.
In water treatment, soda ash is used to bind calcium and magnesium ions to form insoluble calcium and magnesium carbonates, thus making the water soft. This softening method reduces the carbonate hardness but not the permanent hardness. This softening method is relatively cheap.
In the food industry, sodium carbonate is a food additive (E500) used as an acidity regulator, anticoagulant, catalyst and stabilizer, and raising agent. It is one of the components of kansui, a solution of alkaline salts used to give ramen noodles their characteristic taste and texture. It is also used in the production of snus (Swedish-style snuff) to stabilize the pH of the final product.
Sodium carbonate is also used in the production of sherbet powder. The cooling and fizzing sensation is due to an endothermic reaction between the sodium carbonate and a weak acid, usually citric acid, which releases carbon dioxide gases produced when the sherbet is moistened with saliva.
In China, sodium carbonate is used as a substitute for alkaline water, which is used in the production of the traditional 'Cantonese moon' cakes and many other buns and noodles produced in China.
In aquariums, sodium carbonate is used in certain aquarium water pH buffers to maintain the desired pH and carbonate hardness (KH). It is one of the ingredients to produce seawater and to maintain its pH.
In the cleaning industry, soda ash can be used to clean silver by mixing a solution of sodium carbonate and table salt. An aluminum foil of neutral material (glass, plastic or ceramic) and the silver object to be cleaned are immersed in the hot solution. The increase in pH dissolves the aluminum oxide layer on the foil to form an electrolytic cell. The hydrogen ions produced by this reaction reduce the sulfide ions on the silver, thus restoring it to its original form. Sodium carbonate can be used for surface cleaning, either as part of a paste, as a solution or as a scouring powder. It is also included in washing powders, both as a base and as an ingredient.
In cosmetics, soda ash is used in toothpastes, where it acts as a foaming agent and abrasive, and temporarily raises the pH of the mouth. Calcined soda ash is also used as a skin scrub or as a scrub for oily skin.
In leather processing, soda ash is used to soften leather and to produce a smooth, supple effect.
In ceramics, sodium carbonate is used as a wetting agent to reduce the amount of water needed to mix the clay. This produces a soft clay which, when dried or fired, avoids cracks and changes in shape and produces a more cohesive texture.
In bonding, calcined soda is used to increase surface adhesion, and is used to make wet alginate adhere to gelled alginate.
In cotton processing, sodium carbonate is used to neutralize the sulfuric acid required for the acid processing of fluffy cotton seeds.
In metalworking, soda ash is used to neutralize the caustic acid after metal pickling. Sodium carbonate is more convenient to use because less heat is released during neutralization and the reaction takes place under milder conditions.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Caution |
Hazard icons:
|
Danger phrases: H319 Causes severe eye irritation |
Precautionary statements: P264 Wash hands thoroughly after use P280 Wear protective gloves/protective clothing/eye/face protection. P305+P351+P338 IN CASE OF CONTACT WITH EYES: wash gently with water for several minutes. Remove contact lenses if they are present and can be easily done. Continue washing eyes. P337+P313 If eye irritation persists: seek medical advice. |
Related products
(8 other products in the same category)











