|
Parameter |
Attribute |
|
Magnesium sulfate |
Epsom salt (heptahydrate), English salt, Bitter salts, Bath salts |
|
Formula |
MgSO4 |
|
Structure |
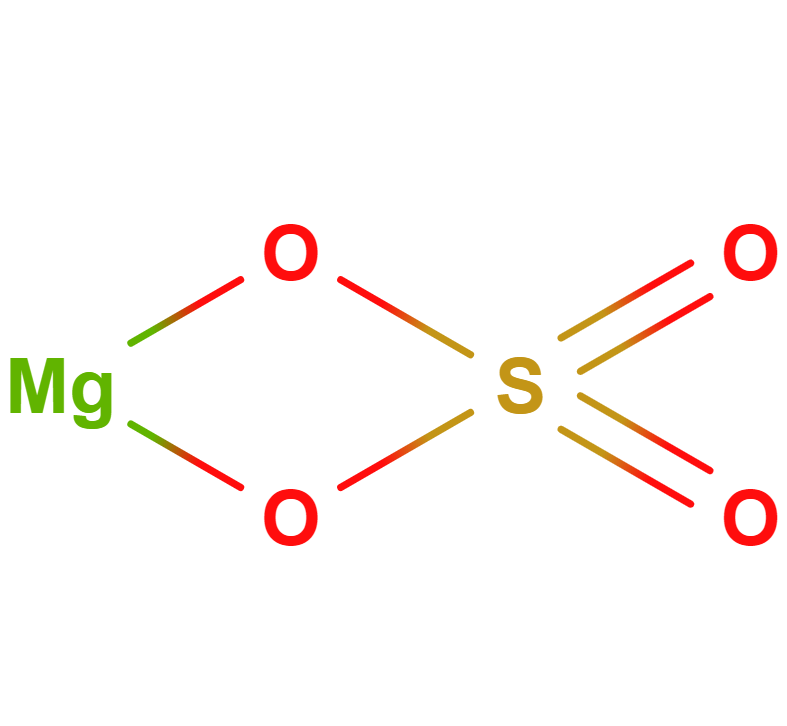  |
|
IUPAC |
Magnesium sulfate |
|
INCI |
MAGNESIUM SULFATE |
|
CAS |
10034-99-8 (heptahydrate) |
|
Molar mass |
246,47 g/mol (heptahydrate) |
|
Density |
1,68 g/cm3 (heptahydrate) |
|
Solubility |
In water: 113 g/100 mL (20 °C) |
Magnesium sulfate is most commonly found as magnesium heptahydrate or as the mineral epsomite. It is more commonly known as Epsom salt. Most commonly found as a fertilizer, but with many other uses, such as deworming.
In horticulture and agriculture, magnesium sulfate is used as a fertilizer. It is used for foliar fertilization and irrigation, dissolves easily and does not leave a residue in water. The high content of magnesium and sulfur in its composition stimulates the photosynthetic process, growth and development of the plant. Can be used as a supplementary fertilizer through the soil, especially in case of magnesium deficiency and during periods of peak demand. Magnesium sulfate is used in horticulture to correct magnesium deficiencies in plants or soils. The advantage of this compound over other magnesium salts is its high solubility, which allows it to be used for foliar fertilization. Compared to other magnesium fertilizers, it has a neutral reaction and has almost no effect on the soil pH reaction. It is commonly used as a fertilizer for gardens (fruit trees, berry plants), greenhouses (cucumbers, tomatoes, peppers), vegetable gardens (lettuce, radishes, potatoes), potted plants and some flowers such as roses. For foliar fertilization, use a solution of 0,1-0,6% (10-60 g/10 l water). For fruit trees and shrubs, spray a 1% solution (100 g/10 l of water). For root fertilization - ornamental plants, flowers, lawns and other plants shall be irrigated with a 0,2% solution (20 g/10 l water).
In cosmetics, magnesium sulfate is used as a base, epsom salt in the manufacture of bath salts. In bath salts, magnesium ions facilitate the absorption of other active ingredients and give the skin a soft feeling. Epsom salt does not react with organic components and does not alter the pH of the bath water or the skin. INCI Key Functions:
- Volume: reduces the visible cosmetic density.
- Viscosity control: increases or decreases the viscosity of cosmetics
In aquariums or aquaculture, magnesium sulfate heptahydrate is used to maintain a constant magnesium concentration in marine aquariums where large quantities of corals are kept. It is absorbed more slowly during calcification, resulting in a slower depletion of reserves. Magnesium sulfate is used to stabilize alkalinity and calcium levels, as calcium changes from an ionic (soluble) form to a solid (non-absorbable) form of calcium carbonate in the presence of magnesium deficiency.
In sports, magnesium sulfate is used as a means of relieving muscle tension and cramps. Epsom salt is used in baths, where magnesium is sorbed through the skin, so that it reaches the muscles and the bloodstream much more quickly than through the digestive tract due to osmotic pressure. Since almost the entire skin of the body is used, the surface area of absorption becomes extremely large, resulting in a very high efficiency. In ICE TUBES Epsom salt is added to reach “soft ice” feeling. The more salt dissolved in the water, the greater the ionic strength, lower the freezing point, and softer the ice that eventually forms. For example, by adding 3-5kg of Epsom salt, it is possible to freeze the entire ice tube water into the slushy consistency of a Slurpie.
In the food industry, magnesium sulfate can be used as an additive in brewing to increase the mineral content required for yeast growth or as a coagulant in tofu production.
In medicine, magnesium sulfate is used as the main raw material for the preparation of intravenous solutions. For the treatment and prevention of convulsions in pregnant women with severe pre-eclampsia or eclampsia. For the treatment and prevention of hypomagnesaemia. For the treatment of polymorphic ventricular arrhythmias (torsades de pointes). Premature labour arrest. Laxative.
In chemistry, anhydrous magnesium sulfate is commonly used as a moisture-absorbing agent in organic synthesis because of its hydrophilic properties. As an electrolyte in the production of copper sulfate.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
|
Signal word: not applicable |
|
Hazard icons: not applicable |
|
Danger phrases: not applicable |
|
Precautionary statements: not applicable |
Related products
(8 other products in the same category)











