CALCIUM CARBONATE 98% (E170 food grade), kg
9.70 €
Calcium carbonate, CAS 471-34-1, INCI CALCIUM CARBONATE, CI 77220, calcium white pigment, calcite, chalk, aragonite, calcite, chalk, lime, limestone.
Parameter | Attribute |
Calcium carbonate | Calcium white pigment, calcium carbonate food grade, aragonite, calcite, chalk, lime, limestone, marble, oyster, pearl, E170 |
Formula | CaCO3 |
Structure | 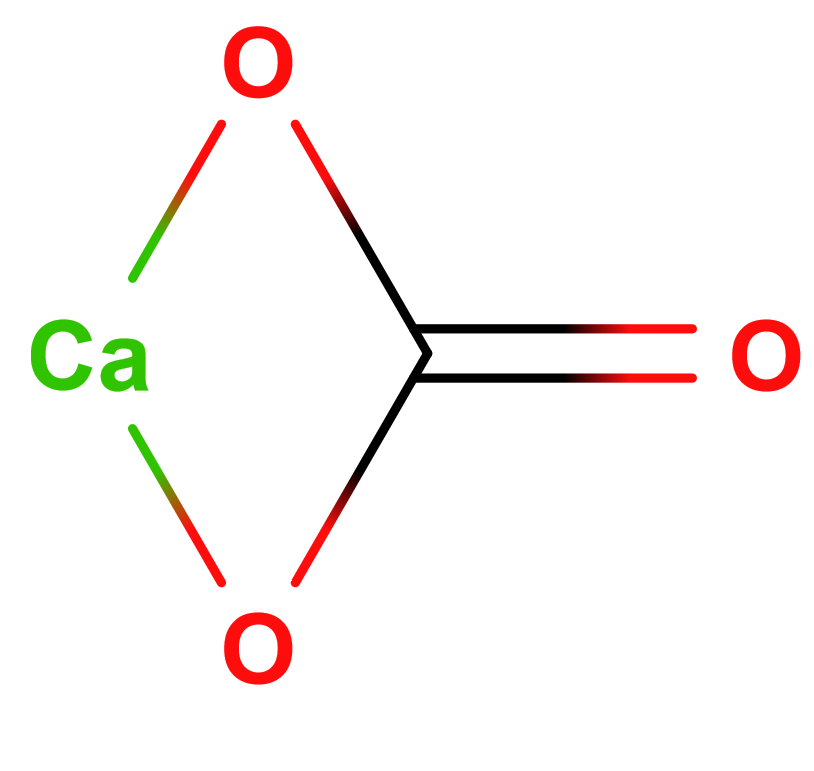 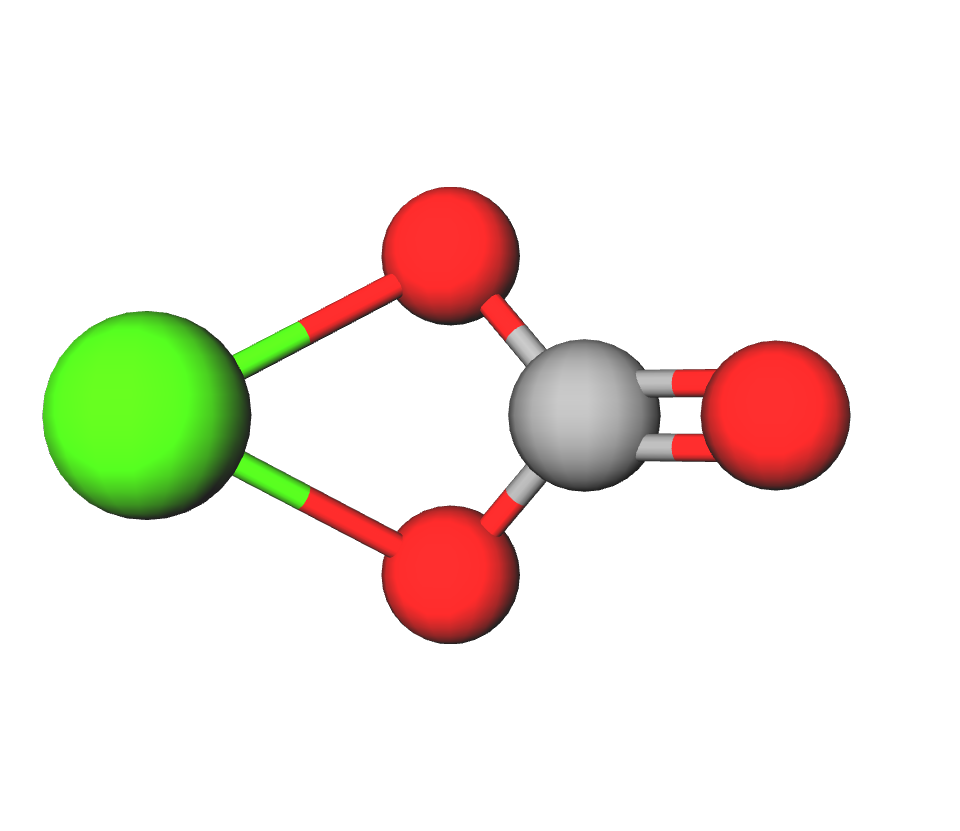 |
IUPAC | Calcium carbonate |
INCI | CALCIUM CARBONATE |
CAS | 471-34-1 |
Molar mass | 100,0869 g/mol |
Density | 2,711 g/cm3 |
Solubility | Soluble in acids (reacts with acidic media), insoluble in alcohols. In water: 0,013 g/l (20 °C) |
Calcium carbonate is a chemical compound with the formula CaCO3. It is a substance commonly found in rocks as the minerals calcite and aragonite (now especially as limestone, which is a type of sedimentary rock composed mainly of calcite) and is a major constituent of eggshells, gastropod shells, crustacean skeletons and pearls. Calcium carbonate is the active constituent of agricultural lime and is formed when calcium ions in hard water react with carbonate ions to form lime deposits. It is used medicinally as a calcium supplement or as an antacid, but excessive use can be dangerous and lead to hypercalcemia and digestive problems.
In the food industry, calcium carbonate is used as a food additive and is labelled E170 with an INS number of 170. It is used as an acidity regulator, viscosity regulator, stabilizer or coloring agent and is approved for use in the EU, USA, Australia and New Zealand. This food additive is added to most milled bread flours, except wholemeal flour, to give the flour a bright white color. Calcium carbonate is also used in sugar production to produce white sugar crystals. It is used in some soya milk and almond milk products as a source of dietary calcium; at least one study suggests that calcium carbonate may be as bioavailable as the calcium in cow's milk. Calcium carbonate is also used as a firming agent in many canned and bottled vegetable products.
In the cosmetic industry, calcium carbonate is used where it is needed to give a white color and to be as safe as possible, e.g. in toothpaste, bath products, make-up products, personal care products, shaving products, oral care products and skin and hair care products. Calcium carbonate is not recommended for use in acidic formulations as it reacts with acids to form soluble compounds and causes the white color to disappear. Attention should be paid to the size of the calcium carbonate fraction: the finer the particle size, the more evenly distributed it is, and the coarser the particle size, the more it tends to be dispersed in the cosmetic product. Calcium carbonate can also be used as an abrasive, a buffer and an oral care agent.
In medicine, calcium carbonate is widely used as an inexpensive dietary calcium supplement for gastric acidity-reducing drugs. It can be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with chronic renal failure). It is used in the pharmaceutical industry as an inert filler for tablets and other drugs.
In animal husbandry, nutritive chalk is used as a feed additive to enrich the diet with the trace element calcium. This method of feeding results in a lower acidity of the feed and in a higher calcium micronutrient content.
In horticulture and aquaculture, food chalk or food limestone is used as a good way to neutralize acidic soils where a particularly pure and clean additive is needed due to the sensitivity of the ecosystem. In aquaculture, food grade calcium carbonate is used in aquariums as a buffer against acidification of the aquarium. It is also an excellent means of neutralizing the acids produced after aquarium cleaning, when the number of organic-degrading bacteria is reduced after filter cleaning.
In laboratories, food grade calcium carbonate is used as a cheaper analogue of laboratory grade but with the same good properties. In schools, it is used as an indicator of acidic reactions (dissolves in acidic media)
In the energy sector, calcium carbonate is used to treat fossil fuel combustion fumes, where it is very effective in binding sulfur and nitrogen oxides (SO2 and NOx) into solid compounds. This method is used in large combustion plants.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- The pictures of the goods may not correspond to the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is of a general nature and may not correspond to the information on the packaging of the product and may not be the exact use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
**- The product complies with the requirements for food additive E170 but is not intended for use as a food additive.
Signal word: not applicable |
Hazard icons: not applicable |
Danger phrases: not applicable |
Precautionary statements: not applicable |
Related products
(8 other products in the same category)










