SODIUM SILICATE (Liquid glass), 50%, L
3.50 €
Sodium silicate solution, CAS 1344-09-8, liquid glass, INCI SODIUM SILICATE.
Parameter | Attribute |
Sodium silicate solution | Liquid glass |
Formula | Na2O(SiO2)x · xH2O |
Structure | 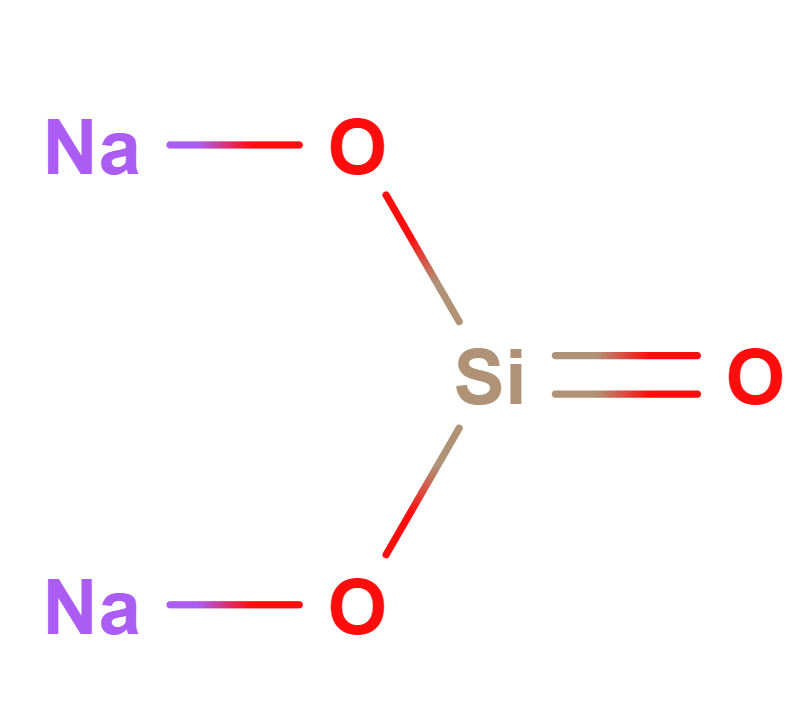  |
IUPAC | Disodium dioxido(oxo)silane |
INCI | SODIUM SILICATE |
CAS | 1344-09-8 |
Molar mass | 122,06 g/mol |
Density | 1,36 g/cm³ at 20 °C |
Solubility | Fully soluble in water |
In construction industry, treating concrete with sodium silicate solution helps reduce porosity in most masonry products. Increased porosity reduces water penetration. A chemical reaction takes place with the excess calcium hydroxide (Ca(OH)2) present in the concrete, which permanently bonds the silicates to the surface, making them much more durable and waterproof. These coatings are known as silicate mineral paints. The liquid glass solution can be used to reinforce the surface of lime concretes simply by coating the concrete surface and allowing the liquid glass to dry. If water gets on the surface during the drying process, it washes away the liquid glass.
In the washing industry, liquid glass is used as an auxiliary component to increase the roughness of the detergent granules. Sodium silicate also protects surfaces from the damaging effects of more aggressive components by coating the surfaces to be washed with a micro-film.
In water treatment, sodium silicate is used as an aluminum coagulant and iron deflocculant in wastewater treatment plants. Sodium silicate binds to colloidal molecules, forming larger aggregates that settle to the bottom and can be filtered out.
In food processing, liquid glass has been used as a preservative for eggs, especially when refrigeration is not possible. Fresh eggs are immersed in a sodium silicate solution (liquid glass). The sodium silicate coats the eggs and prevents bacteria from gaining access to the eggs. Eggs treated using this method can be kept fresh for up to five months.
In automotive repair, sodium silicate is also used as an exhaust system joint and crack sealant for the repair of mufflers, resonators, tailpipes and other exhaust components, with or without fiberglass reinforcement strips. For this purpose, sodium silicate (60-70%) is normally mixed with kaolin (40-30%), an aluminum silicate mineral, to make the sodium silicate 'glued' joint opaque. Sodium silicate acts as a high-temperature adhesive, while kaolin is used only as a high-temperature colorant. Sodium silicate can be used to fill gaps between cylinder head gaskets. It is most commonly used for aluminum alloy cylinder heads which are susceptible to heat-induced surface deformation. "The 'liquid glass' (sodium silicate) is added to the system via a radiator and allowed to circulate. The sodium silicate is suspended in the cooler until it reaches the cylinder head. At temperatures of 100-105 °C (212-221 °F), the sodium silicate loses water molecules to form glass seals with a melting point exceeding 810 °C (1490 °F). Sodium silicate is used in combination with magnesium silicate to produce a mounting paste. When dissolved in water, both sodium silicate and magnesium silicate form a thick paste-like material that is easy to handle. When the exhaust system of an internal combustion engine is heated up to its operating temperature, the heat displaces any excess paste. The remaining silicate compounds have glass-like properties, making it a temporary, brittle repair. Sodium silicate can be used as a component of regenerative pastes.
In the manufacture of refractories Sodium silicates have the property of swelling (expanding) when exposed to heat. The expanding sodium silicate protects the coated material against heat. Liquid glass is used as a binder for solid materials such as vermiculite and perlite. When liquid glass is mixed with the above-mentioned lightweight fillers, the resulting filler can be used for heavy, high-temperature refractory insulation boards, passive fire protection and high-temperature insulating materials, e.g. for the formation of pipe insulation. Mixed with finely divided mineral powders such as Vermiculite dust, high temperature adhesives can be produced.
In geology, sodium silicate is often used in drilling fluids to stabilize borehole wells and prevent borehole walls from cracking. It is particularly useful when boreholes penetrate clay structures containing soluble clay minerals such as smectite or montmorillonite. Sodium silicate with additives was injected into the ground to harden it and thus prevent further leakage of highly radioactive water from the Fukushima Daiichi nuclear power plant in Japan in 2011.
In ceramics, sodium silicate is used as a deflocculant in molding to reduce viscosity and the amount of water needed to soften the clay. It is also used to create a fissure effect in ceramics. It is also the main 'magic water' agent used in joining clay parts, especially if the moisture content of the two parts is different.
In dyeing industry, sodium silicate solution is used as a fixative for hand dyeing with reactive dyes that require a high pH to react with the textile fiber.
In schools it is used for the rapid growth of crystals and the formation of colored precipitates.
In the manufacture of firearms cartridges (magazines), the use of the adhesive properties of sodium silicates can be traced back to the manufacture of black powder revolver paper cartridges by Colt's Manufacturing Company from 1851 to 1873, particularly during the American Civil War. Sodium silicate was used to burn the nitrated paper tightly to form a conical paper cartridge to hold the black powder, as well as to cement the lead ball or conical bullet in the open end of the paper cartridge. Such sodium silicate cemented paper clips were placed in the cylinders of the revolvers, thus speeding up the cap-and-ball loading of the black powder revolvers. This use essentially culminated in the introduction of Colt revolvers using copper cases from 1873.
Important: Add the item to your basket, fill in the recipient's details and confirm your order. Thank you!
To save your precious time, we will deliver your order to your address at a time convenient for You!
*- Pictures of the goods may not reflect the actual appearance, color, assembly or shape of the goods and their packaging. The information in the product description is general and may not correspond to the information on the packaging of the product and may not be accurate as to the use of the product. The information given on the stocks and prices of goods may, in certain cases, differ from the actual prices and stocks of goods
Signal word: Danger |
Hazard icons:
|
Danger phrases: H315 Irritating to skin H319 Causes severe eye irritation |
Precautionary statements: P262 Prevent contact with skin or clothing P280 Wear protective gloves/protective clothing/use eye protection/face protection. P303+P361+P353 IN CASE OF CONTACT ON SKIN (or hair): remove immediately any contaminated clothing. Wash skin with water [or spray]. P305+P351+P338 IN EYES: wash gently with water for several minutes. Remove contact lenses, if present and if easy to do so. Continue washing eyes. |
Related products
(8 other products in the same category)











